A 24-year-old woman with an otherwise unremarkable medical history presented to another health-care institution because of neck pain. Cervical magnetic resonance imaging (MRI) demonstrated a hypointense marrow-infiltrating lesion involving the T1 vertebral body (Figs. 1-A and 1-B). A core biopsy was obtained (Fig. 2).
It was thought that complete resection of the lesion would likely be associated with considerable morbidity, so the patient was managed with denosumab with improvement in clinical symptoms and imaging evidence of a stable tumor size on computed tomography (CT) for >3 years, although there was a gradual increase in sclerosis and compression deformity of T1 (Figs. 3, 4-A, and 4-B).
Recurrent neck pain developed 9 months after the discontinuation of denosumab. Physical examination at that time revealed tenderness to palpation over the left cervical paraspinal region, impaired sensation corresponding to the left T1 nerve root, and slightly decreased left-sided grip strength. CT scans that were made 16 months after the discontinuation of denosumab revealed progression of a calcified T1 lesion with vertebral body involvement and expansion of the calcified transverse process, displacing the apex of the left lung (Figs. 5-A and 5-B). MRI scans showed a larger mass compared with previous MRI scans, with areas of low signal in the periphery on all pulse sequences, corresponding with the calcification on CT scans (Figs. 6-A, 6-B, and 6-C).
Approximately 16 months after completing denosumab treatment, the patient underwent a 2-stage procedure for resection of the tumor. Stage 1 included C7-T1 laminectomy with resection of all posterior elements of T1, removal of the T1 lamina pedicle transverse process, and removal of the paraspinal mass, followed by posterior arthrodesis from C4 to T4. Stage 2 was performed 2 days later and included complete corpectomy of T1 with anterior arthrodesis from C7 to T2.
Histological examination of the resection specimen is shown in Figs. 7-A, 7-B, and 7-C.
At 1 year of follow-up, the patient was doing well, with substantial recovery of grip strength in the left hand and resolution of the neck pain. Imaging studies at that time revealed no evidence of tumor recurrence and solid osseous fusion from C4 to T4.
The patient was diagnosed with residual or recurrent stromal tumor cells of a giant cell tumor of bone.
Proceed to Discussion >>
Reference: Zhang Y, Ilaslan H, Krishnaney AA, Bauer TW. Morphological transformation of giant-cell tumor of bone after treatment with denosumab: a case report. JBJS Case Connect. 2016 Sep 14;6(3):e74.
Giant-cell tumor of bone (GCTB) is a benign, locally aggressive lytic neoplasm that accounts for approximately 4% to 5% of all primary bone tumors. Histologically, it is composed of a proliferation of oval, or spindled, mononuclear cells admixed with uniformly distributed osteoclast-type giant cells. It is now thought that the mononuclear cells are neoplastic and clonal, exhibiting specific H3F3A driver mutations. These clonal mononuclear cells may show phenotypic markers of osteoblasts such as RUNX2 (runt-related transcription factor 2) and SATB2 (special AT-rich sequence-binding protein 2) and express RANKL (receptor activator of nuclear factor κ-B ligand), which recruits the osteoclast precursor cells and induces the differentiation of osteoclast-type multinucleated giant cells in GCTB. The abundant osteoclastic giant cells lead to bone reabsorption and allow growth of the tumor. In June 2013, the U.S. Food and Drug Administration (FDA) approved the use of denosumab, a monoclonal antibody antagonist of RANKL, for the management of patients who have unresectable GCTB or those in whom resection would likely result in severe morbidity. By binding to RANKL, denosumab prevents the RANK-RANKL interaction and thus the formation of osteoclastic giant cells in the region of the tumor. A spectrum of histological changes after denosumab treatment has been described recently, with the main features including a reduction or depletion of osteoclastic giant cells and an increase in woven-bone formation.
This case confirms the findings of previous studies that denosumab treatment can induce radiographic and histological changes in GCTB. As described in those reports, the resection specimen was notable for the absence of osteoclastic giant cells but a marked increase in bone formation in the mononuclear component of the tumor that progressed. A spectrum of histological changes has been described after treatment with denosumab. Several reports have described a storiform growth pattern of spindled cells with a morphological appearance similar to that of benign fibrous histiocytoma. Others have suggested an aggressive appearance of osteoid similar to the morphology of an osteosarcoma. Wojcik et al. recently suggested that the histological appearance may relate to the length of denosumab treatment, with early lesions showing a cellular spindled stroma and with lesions excised later showing more prominent bone formation reminiscent of fibrous dysplasia or low-grade central osteosarcoma. In the case of this patient, the multinuclear osteoclastic giant cells were depleted and replaced by newly formed woven bone, deposited in interconnected strands with prominent osteoblastic rimming and intervening loose fibrovascular stroma, features similar to those of an osteoblastoma. Areas of spindled stromal cells resembling a benign fibro-osseous lesion were simultaneously present.
While denosumab has proven to be effective for reducing the number of osteoclastic giant cells by interfering with osteoclastic differentiation, it does not necessarily eliminate neoplastic stromal cells. For example, Girolami et al. demonstrated the same H3F3A-mutated mononuclear cell populations both before and after treatment of a GCTB with denosumab. Previous in vitro experiments also showed that stromal cells continued to proliferate once the tumor was no longer exposed to denosumab. It has been suggested that these neoplastic stromal cells are osteoblast-lineage cells, which express osteoblast and preosteoblast markers, a concept supported by the increased osteoid matrix and woven-bone formation noted in this patient. However, it is unclear whether denosumab induces a partial maturation of neoplastic stromal cells toward osteoblastic maturation, leading to osteoid matrix deposition and woven-bone formation, as suggested by Girolami et al., or whether the increased bone formation reflects a shift in the balance away from resorption and toward bone formation by depleting the osteoclastic giant cells in the GCTB. It is difficult to make strong treatment recommendations on the basis of a case report, but this case, as well as a growing understanding of the mechanism of action of denosumab, suggest that this treatment may be effective for reducing the osteoclastic giant cells and limiting tumor size but should not necessarily be expected to result in necrosis of the entire GCTB. Continued proliferation of the neoplastic component of the GCTB may continue, so close follow-up of patients who have been managed with denosumab seems appropriate. Pathologists, radiologists, and surgeons need to be aware of this post-treatment effect to avoid misdiagnosis of recurrent GCTB as an osteoblastoma or other fibro-osseous lesion. In addition, it appears that treatment with denosumab alone may only partially address the therapeutic need of the patient.
Reference: Zhang Y, Ilaslan H, Krishnaney AA, Bauer TW. Morphological transformation of giant-cell tumor of bone after treatment with denosumab: a case report. JBJS Case Connect. 2016 Sep 14;6(3):e74.
What is the diagnosis?
Brown tumor of hyperparathyroidism
Residual or recurrent stromal tumor cells of a giant cell tumor of bone
Chondroblastoma
“Solid” aneurysmal bone cyst
Osteoblastoma

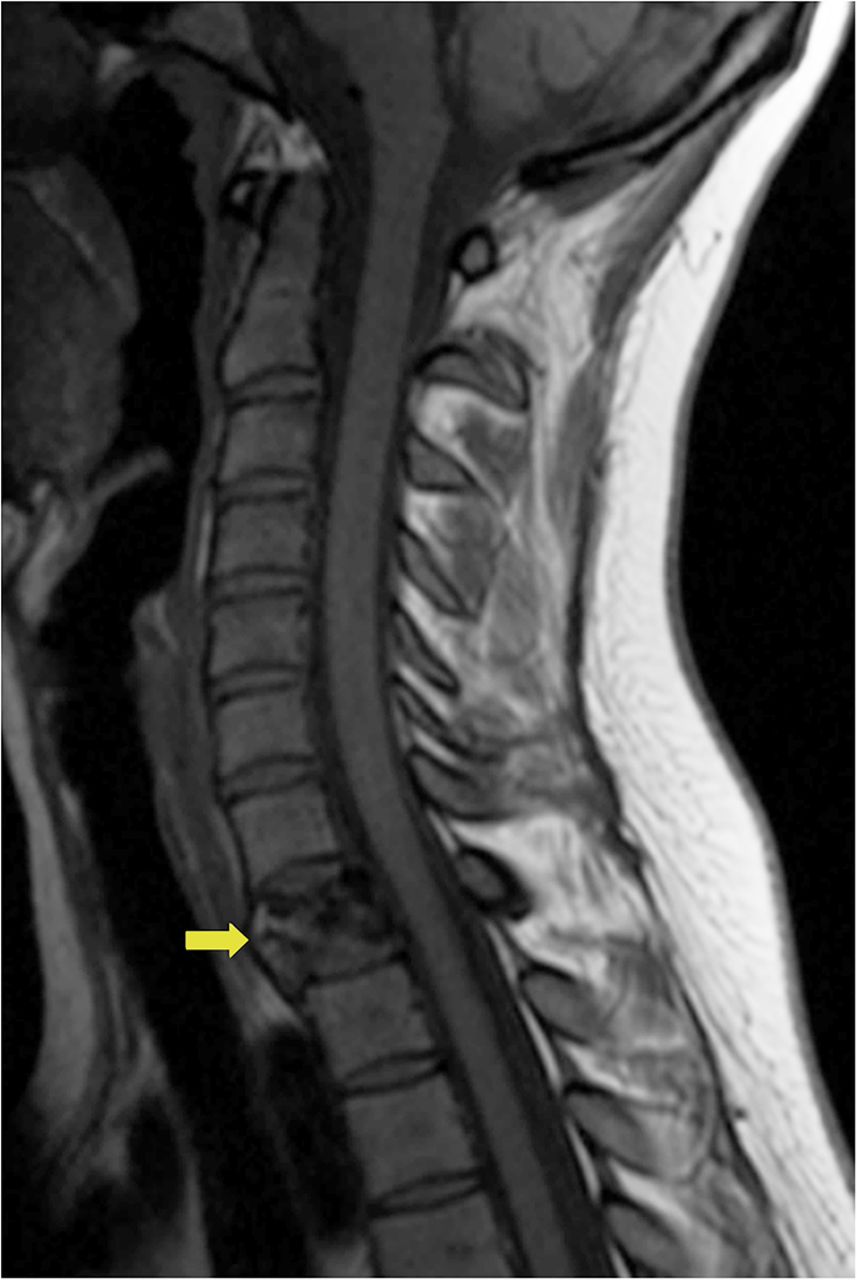
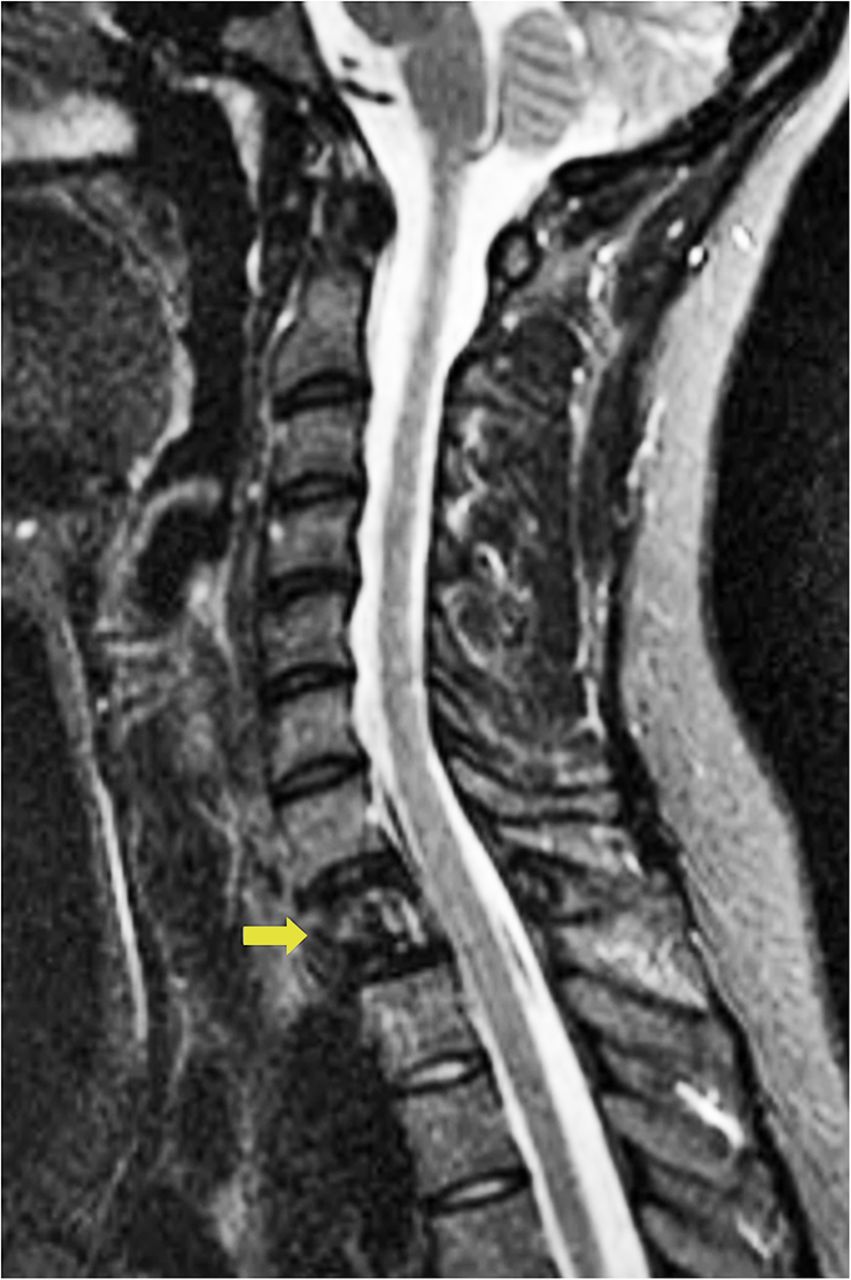
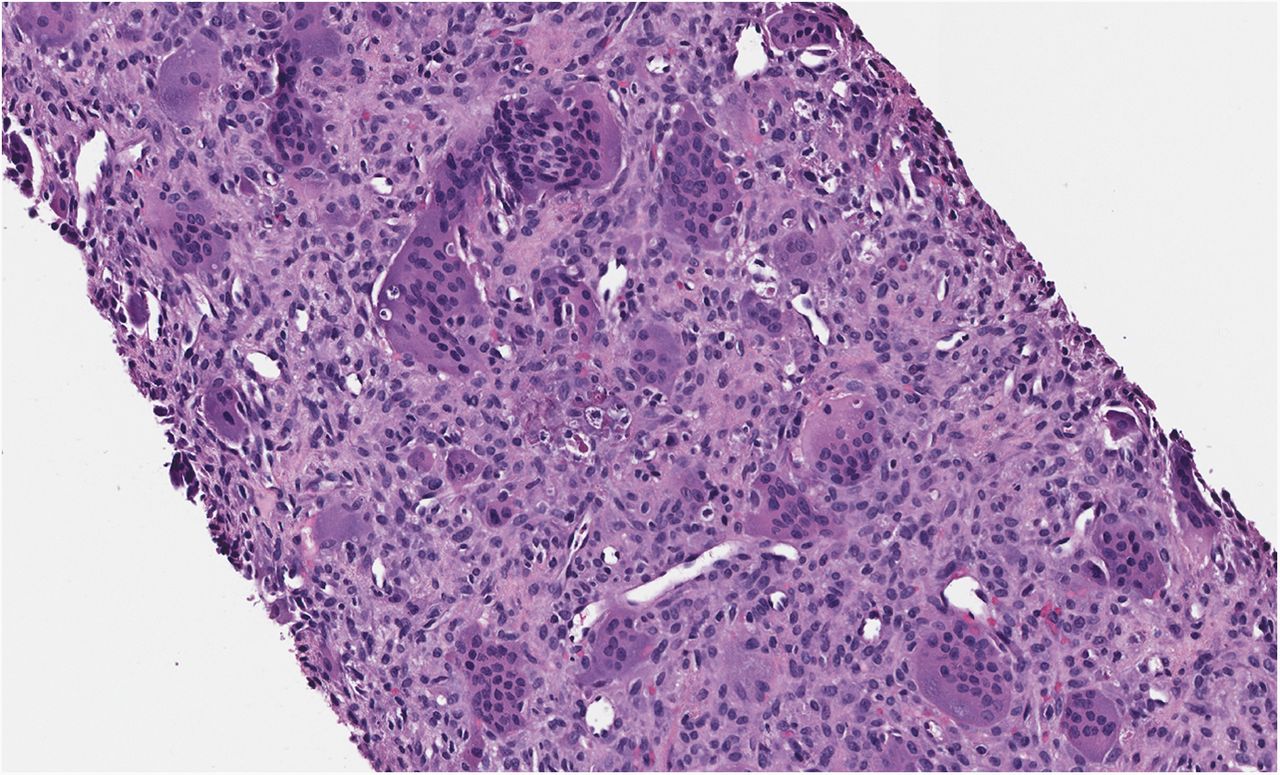

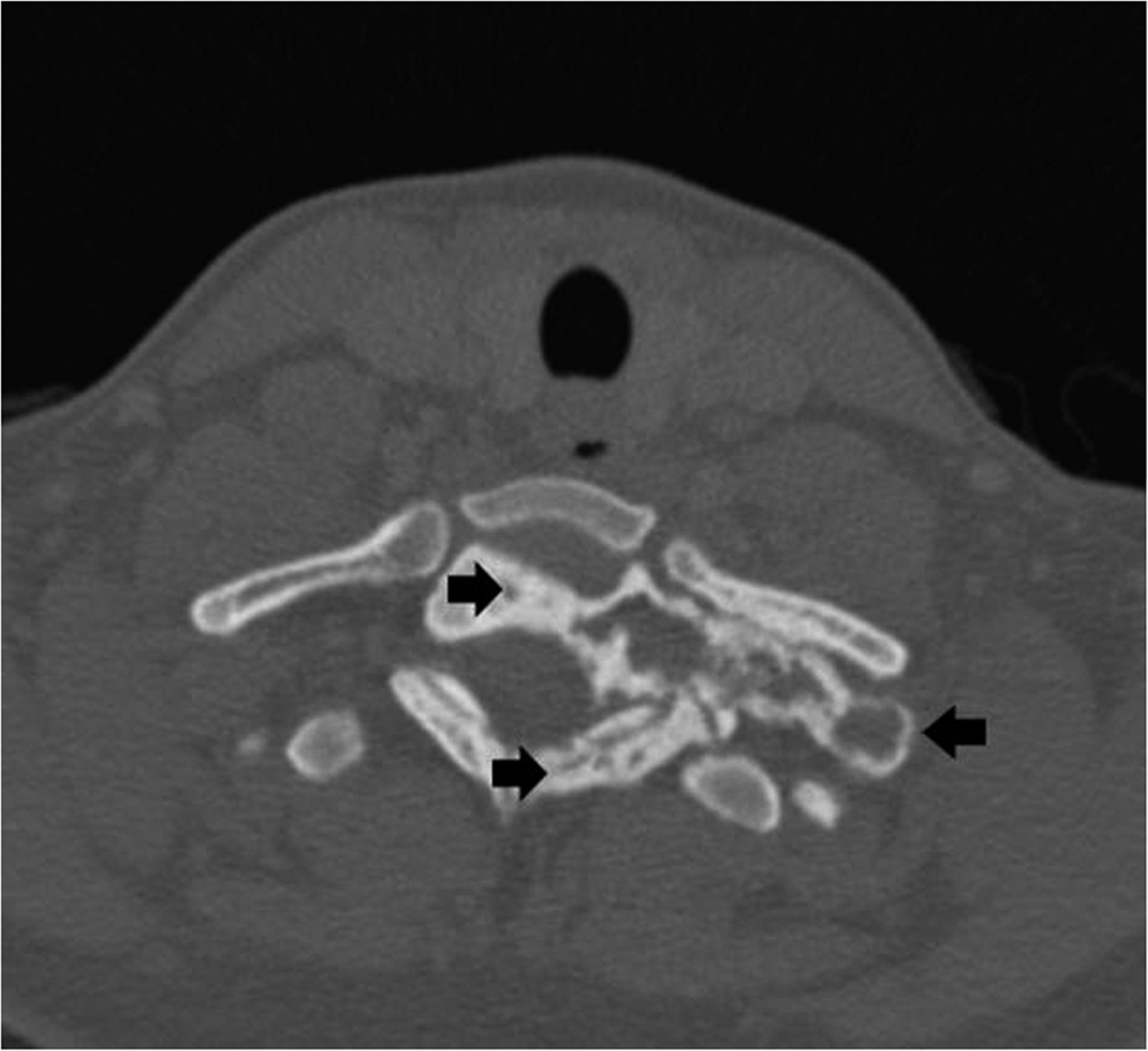
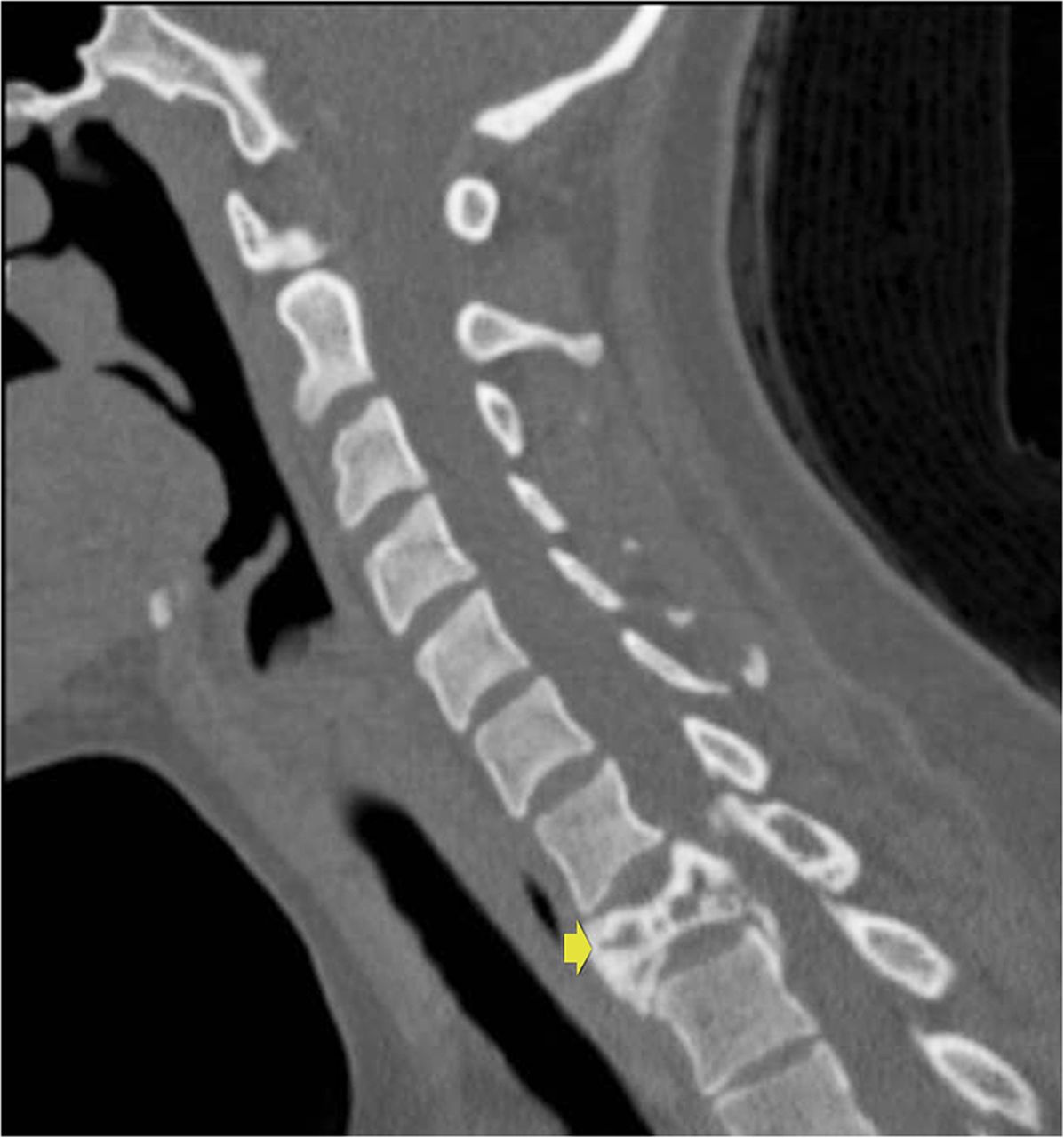
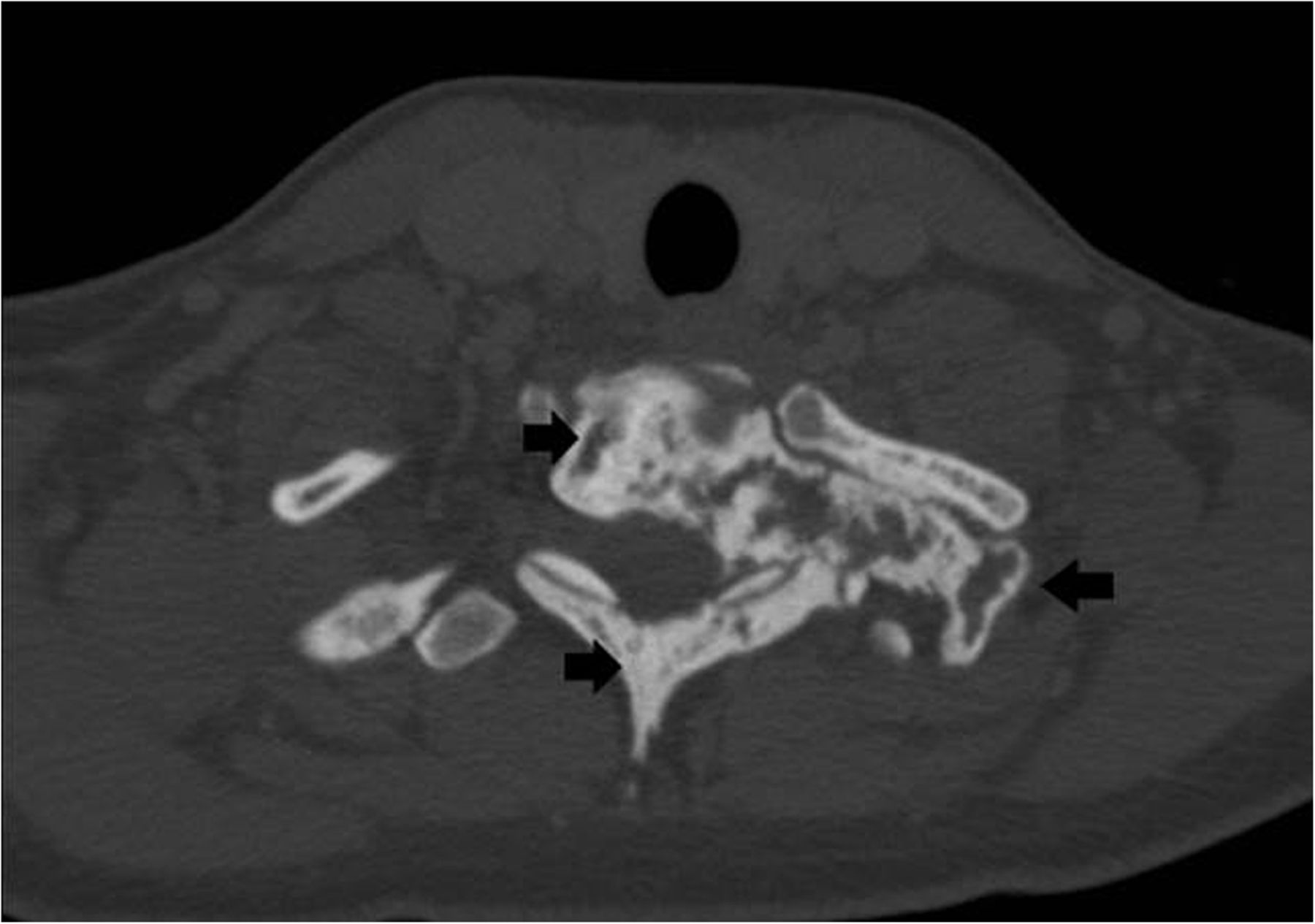


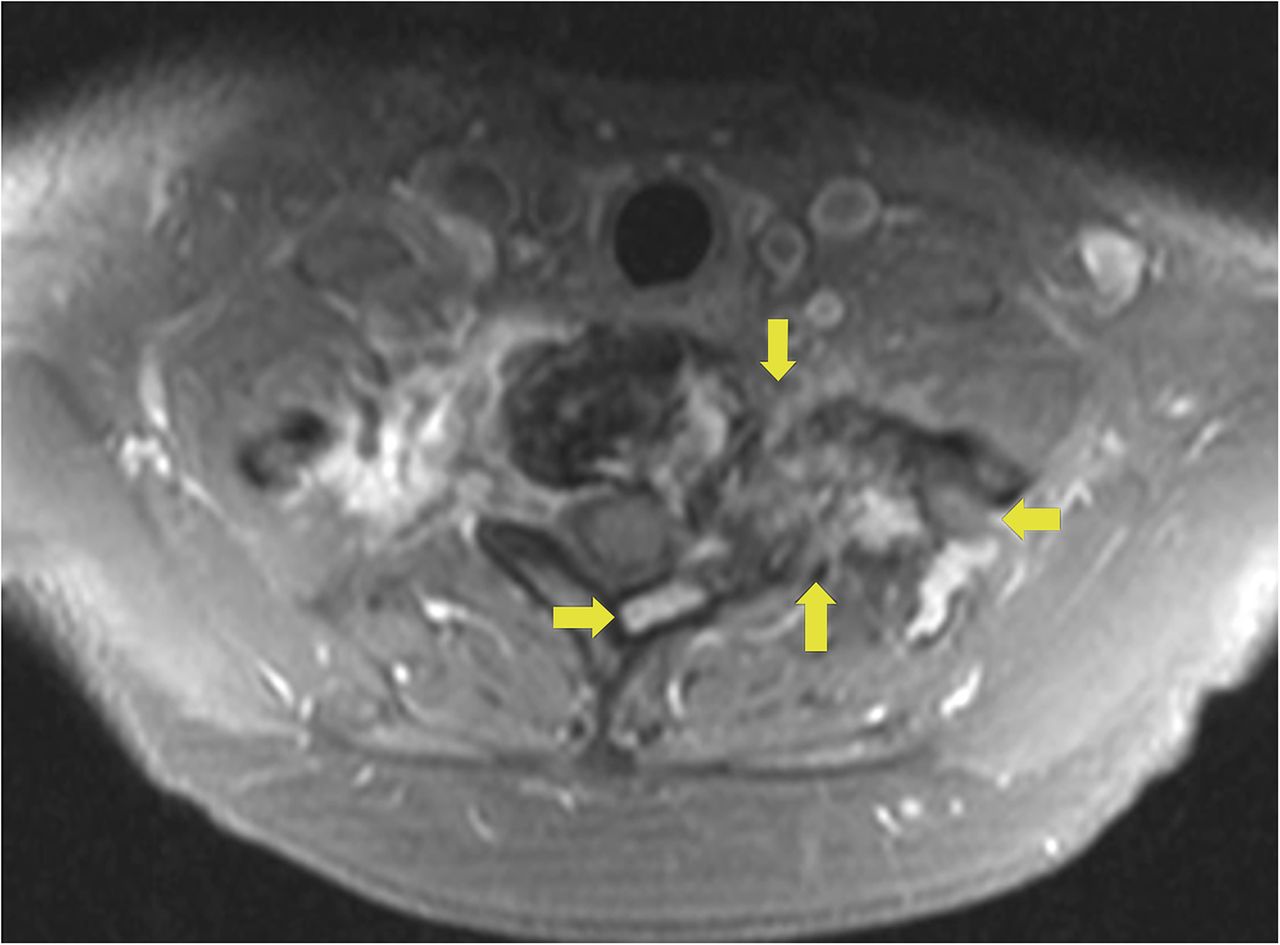
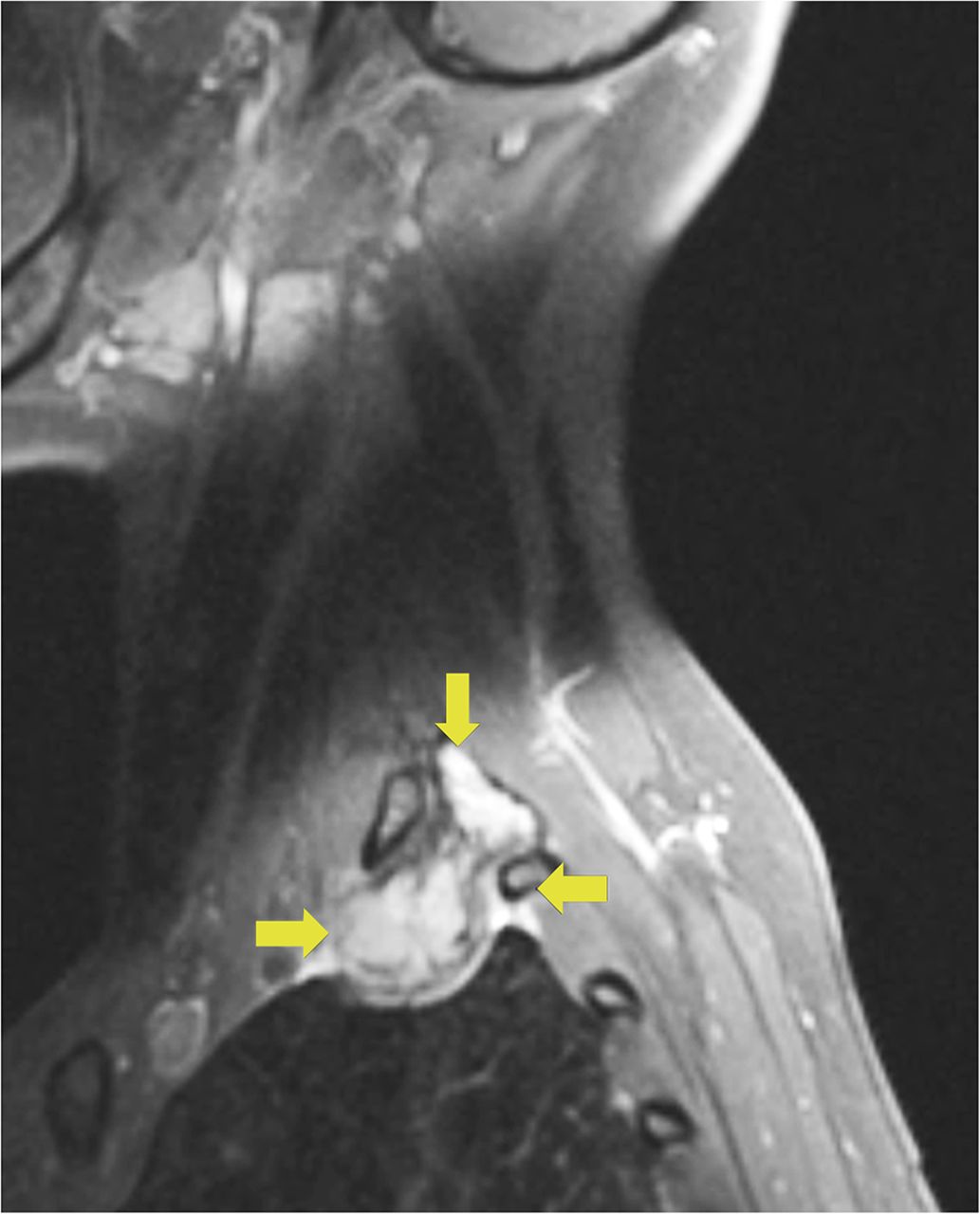
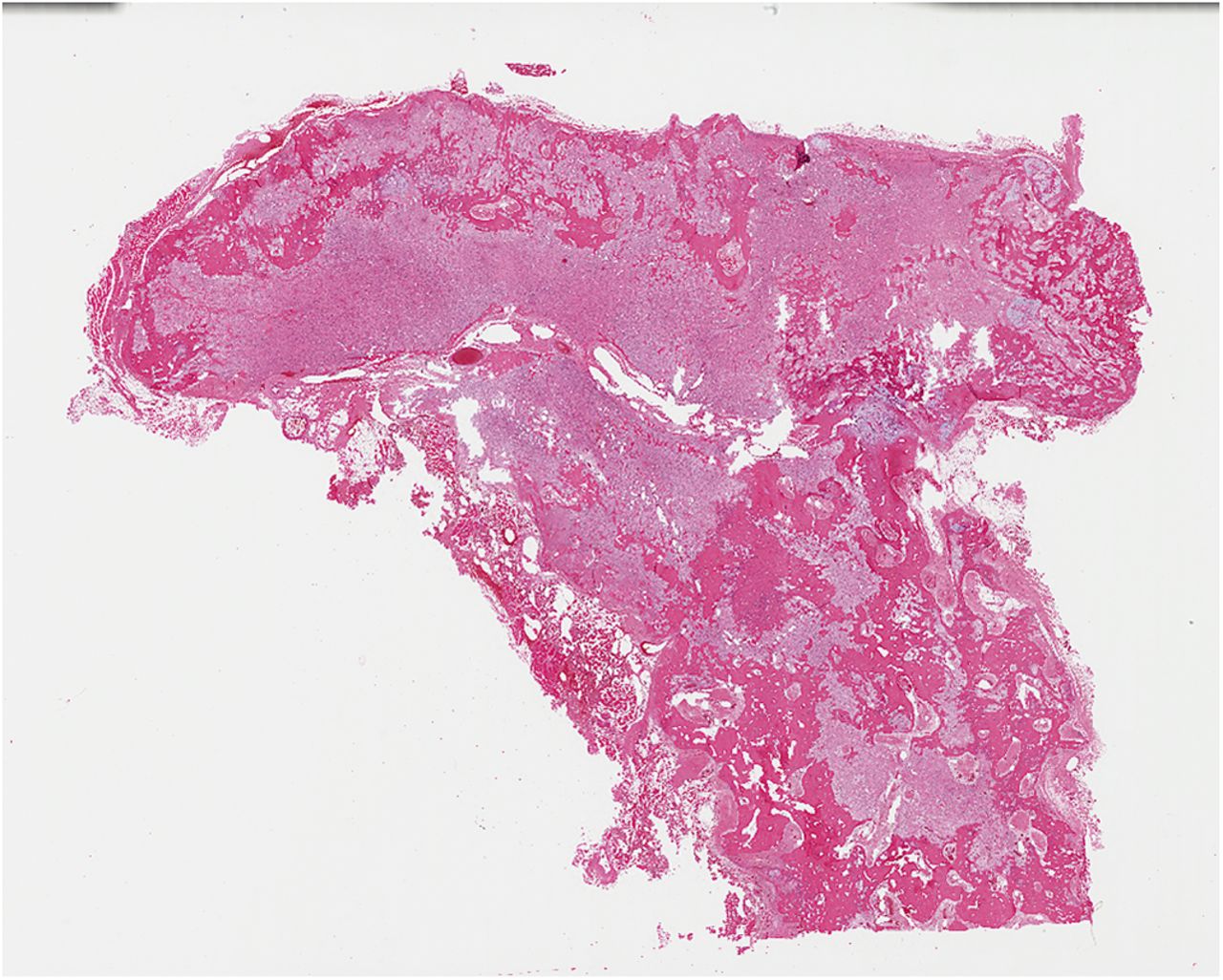
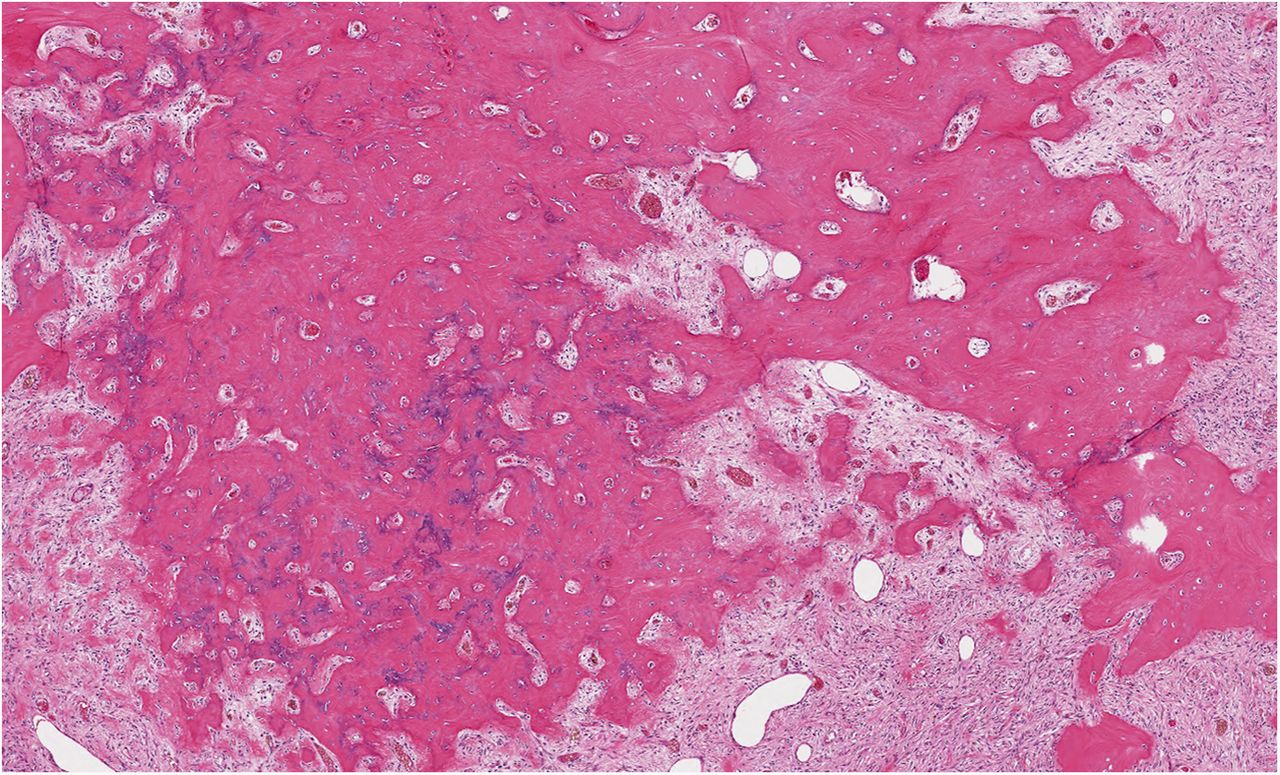
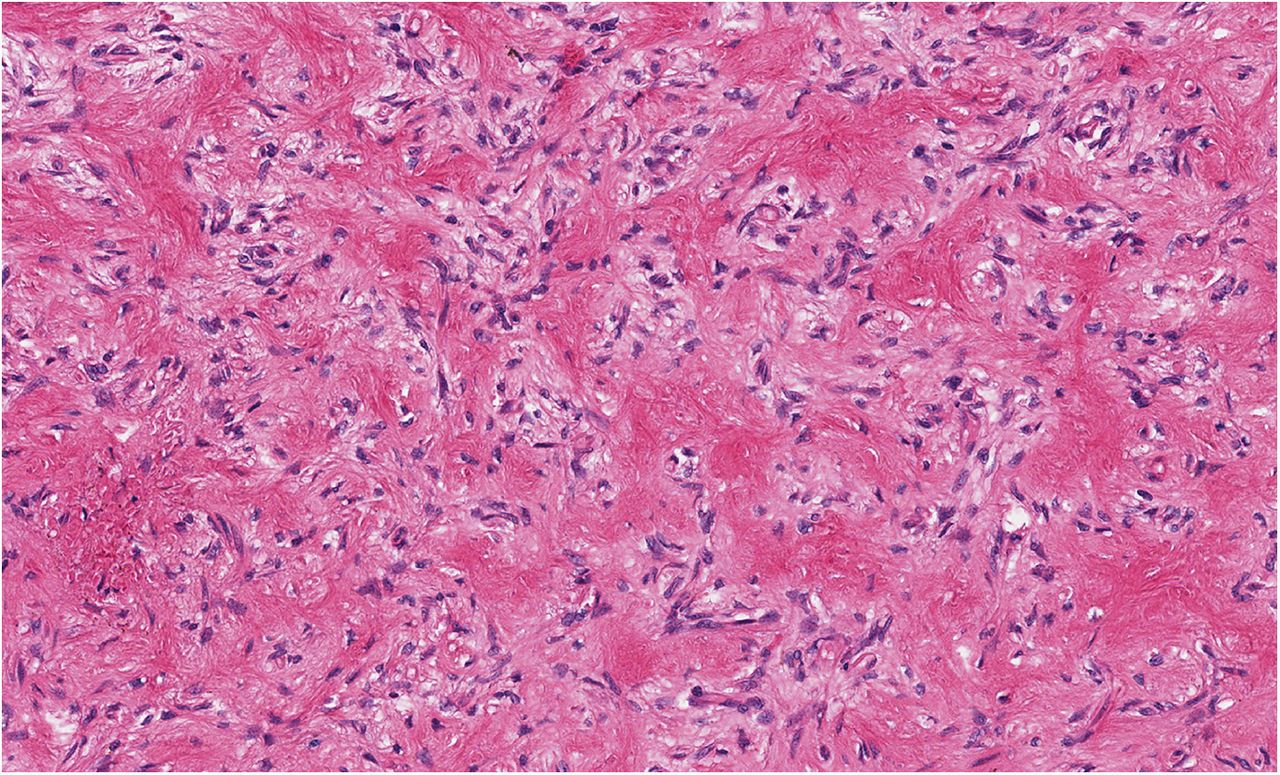
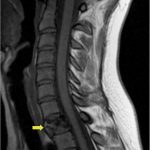 Fig. 1-A
Fig. 1-A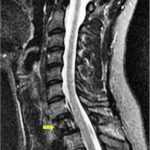 Fig. 1-B
Fig. 1-B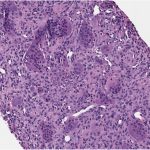 Fig. 2
Fig. 2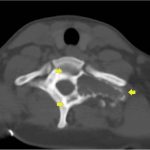 Fig. 3
Fig. 3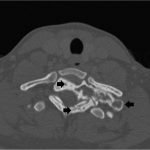 Fig. 4-A
Fig. 4-A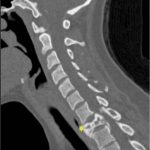 Fig. 4-B
Fig. 4-B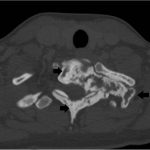 Fig. 5-A
Fig. 5-A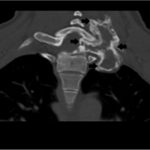 Fig. 5-B
Fig. 5-B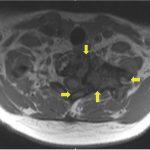 Fig. 6-A
Fig. 6-A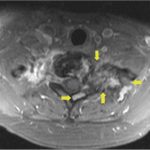 Fig. 6-B
Fig. 6-B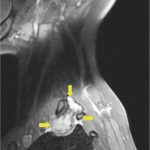 Fig. 6-C
Fig. 6-C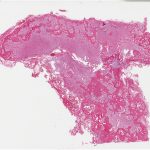 Fig. 7-A
Fig. 7-A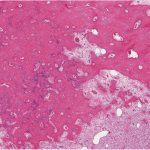 Fig. 7-B
Fig. 7-B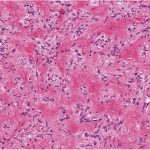 Fig. 7-C
Fig. 7-C