A 50-Year-Old Woman with Long-Term Weakness, Swelling, and Stiffness
September 20, 2017
A 50-year-old woman presented with a 10-year history of diffuse bone pain, weakness, stiffness, and swelling. She had undergone previous medical evaluation and had received a presumptive diagnosis of fibromyalgia. She subsequently had experienced a hip fracture from a low-energy mechanism. This fracture had progressed to nonunion, requiring a bone-grafting procedure. Two years later, she had been diagnosed with osteomalacia, and was no longer ambulatory secondary to progressively worsening severe pain. Despite being reliant on a wheelchair, she had experienced a second left hip fracture the next year. Serum analysis had shown hyperphosphaturia, hypophosphatemia, and elevated levels of intact parathyroid hormone (iPTH) and fibroblast growth factor 23 (FGF-23). A computed tomography (CT) had been performed and had shown a subcutaneous mass in the proximal aspect of the right thigh overlying the intertrochanteric region, measuring 3.0 × 2.7 × 3.4 cm.
Serum FGF-23 had been measured to be 540 RU/mL (normal, ≤180 RU/mL). CT that had been performed later in the same year was notable for a subcutaneous lesion 1.7 cm in diameter in the anterolateral aspect of the proximal portion of the right thigh overlying the lesser trochanter, as well as for a 1.5-cm subcutaneous left buttock mass. In the subsequent year, excision of these masses had been performed.
A follow-up positron emission tomography (PET)-CT scan had been performed 10 months after the surgical excision and had shown no signs of hypermetabolic activity in the field of view (head to midthigh). Two years after the PET-CT scan, the patient underwent a repeat octreoscan with a larger field of view, which showed foci of increased radiotracer uptake in the right talus (Fig. 1). Magnetic resonance imaging (MRI) confirmed a sizable lesion occupying the talus (Figs. 2 and 3). Additional cross-sectional imaging showed diffuse pelvic and femoral insufficiency fractures. CT-guided biopsy of the talar lesion was followed by intralesional excision with curettage and high-speed burring and implantation of a rebar-and-cement construct (Fig. 4). Histopathology is shown in Fig. 5.
The patient was diagnosed with a metastatic phosphaturic mesenchymal tumor. After surgery, the patient’s clinical course improved. She gradually progressed from protected weight-bearing to ambulation with a controlled ankle motion walker boot and a walker. By 4 months after surgery, she was able to walk independently for brief intervals. At 1 year after surgery, she reported a progressive improvement in pain. Foot radiographs at that time demonstrated persistent osteoporosis but no evidence of local tumor recurrence. Interestingly, the patient’s biochemical profile did not mirror the clinical improvement postoperatively. At the latest follow-up, the serum phosphate level had decreased to 1.5 mmol/L, and the alkaline phosphatase level had increased to 331 IU/L.
Proceed to Discussion >>Reference: Aziz KT, McCarthy EF, Morris CD. Oncogenic osteomalacia secondary to a metastatic phosphaturic mesenchymal tumor in the talus: A case report and review of the literature. JBJS Case Connect. 2017;7(2):e40.
Oncogenic osteomalacia is a well-known paraneoplastic syndrome associated with solid tumors, including phosphaturic mesenchymal tumors (PMTs). The rarity of PMTs contributes to challenges in diagnosis and treatment. In our patient, there was an approximate 10-year delay between the onset of symptoms and diagnosis, which is even longer than the previously reported diagnostic delays of 5 to 7.2 years. Further confounding the difficulty in treating PMTs is the difficulty in localizing the tumor, which has been reported to take as long as 20 years.
Diagnosing lesions in the distal aspect of the lower extremities can be challenging because they are not included in most routine staging scans. Serial imaging for recurrence of PMTs may include octreoscans, CT, PET-CT, and whole-body MRI. CT and MRI are limited in localizing PMTs. Augmented imaging techniques using radiolabeled somatostatins (octreoscans) facilitate tumor localization because PMTs frequently express somatostatin receptors. A previous study showed that PET-CT may be an invaluable supplemental imaging modality in cases in which an octreoscan fails to localize the tumor; however, as with our patient, it is critical to adjust the PET-CT protocol by expanding the scanning area to include the hands and feet. Lee et al. recently reported on 66 PMTs and found that most (82%) of the tumors were FGF receptor 1 (FGFR1)-positive on immunohistochemical staining, thereby offering a diagnostic tool in the evaluation of these tumors. In addition, they reported a novel FN1-FGF1 fusion gene in a subset of the studied PMTs.
Metastatic PMTs are exceptionally rare. Only 3 of 32 patients described by Folpe et al. had the histologic appearance of malignant disease, and only 1 of these had metastases. Only 1 of 17 cases of PMT described by Wang et al. had evidence of metastatic disease. These series reported metastases to the lung and the tibia. A recent study has described the frequency of metastasis and recurrence to be <5%. Although metastatic skeletal disease occurs in approximately 30% of patients with malignancy, metastasis to the hands or feet (acrometastasis) is uncommon. The incidence of acrometastasis has been reported to range from 0.007% to 0.3%, with metastatic disease of the hand being 3 times more common than metastases to the foot. This patient is notable for a rare neoplasm, malignant in <5% of cases, with confirmed acrometastasis.
The standard of care for treating PMTs is surgical excision and careful monitoring for recurrence. However, in cases in which the patient is not a good surgical candidate or when the lesion is surgically inaccessible, alternative treatments have been described. Prior literature has shown that cinacalcet, a calcimimetic, decreases the phosphaturic effect of FGF-23 in disorders of FGF-23-mediated hypophosphatemia. In prior literature, octreotide therapy has shown efficacy, which is likely secondary to modulation of FGF-23 secretion by binding with somatostatin receptors. These receptors have been found in most PMTs and are identified with octreoscans; however, the systemic effects of octreotide therapy and the absence of somatostatin receptors in some variants must be considered. A human monoclonal anti-FGF-23 antibody, KRN23, is a novel therapy currently being studied for patients with PMTs. Previous studies have shown that KRN23 substantially increased the maximum renal tubular threshold for phosphate reabsorption to glomerular filtration rate (TmP/GFR), serum phosphorus, and 1,25 dihydroxyvitamin D3 (1,25[OH]2D3) levels. Given the proposed role of FGF-23 in oncogenic osteomalacia with PMTs, this therapy could benefit patients with recurrent or inoperative disease.
This case report highlights the need for tenacity and diagnostic vigilance when the patient continues to deteriorate despite appropriate interventions. A PMT should be on the differential diagnosis list for a patient with unexplained osteoporosis. When the laboratory workup, including serum phosphorus and PTH testing, suggests osteomalacia, testing for serum FGF-23 should be considered. Elevated FGF-23 should trigger screening for localization of a primary tumor. If the clinical status of a patient who has undergone resection with negative margins does not improve, suspicion should be raised immediately for recurrent lesions, including metastatic lesions. Our patient continued to have symptoms and atypical biochemical values despite negative margins on surgical resection. Although PMTs have been reported to have a rate of metastasis of <5%, this case report shows the value of longitudinal follow-up with serial biochemical, radiographic, and clinical monitoring. In the rare cases of reported metastasis, a higher mitotic rate and the presence of the Ki-67 antigen often have been observed. PMTs have been referred to as “strange tumors that occur in strange places,” and as evidenced by this case report, they require a heightened awareness of the disease process in combination with timely biochemical, radiographic, and clinical assessment for optimal treatment.
Reference: Aziz KT, McCarthy EF, Morris CD. Oncogenic osteomalacia secondary to a metastatic phosphaturic mesenchymal tumor in the talus: A case report and review of the literature. JBJS Case Connect. 2017;7(2):e40.
What is the diagnosis?
Synovial sarcoma
Metastatic phosphaturic mesenchymal tumor
Metastatic adenocarcinoma consistent with lung origin
Osteoporosis with multiple insufficiency fractures
Brown tumor of hyperparathyroidism

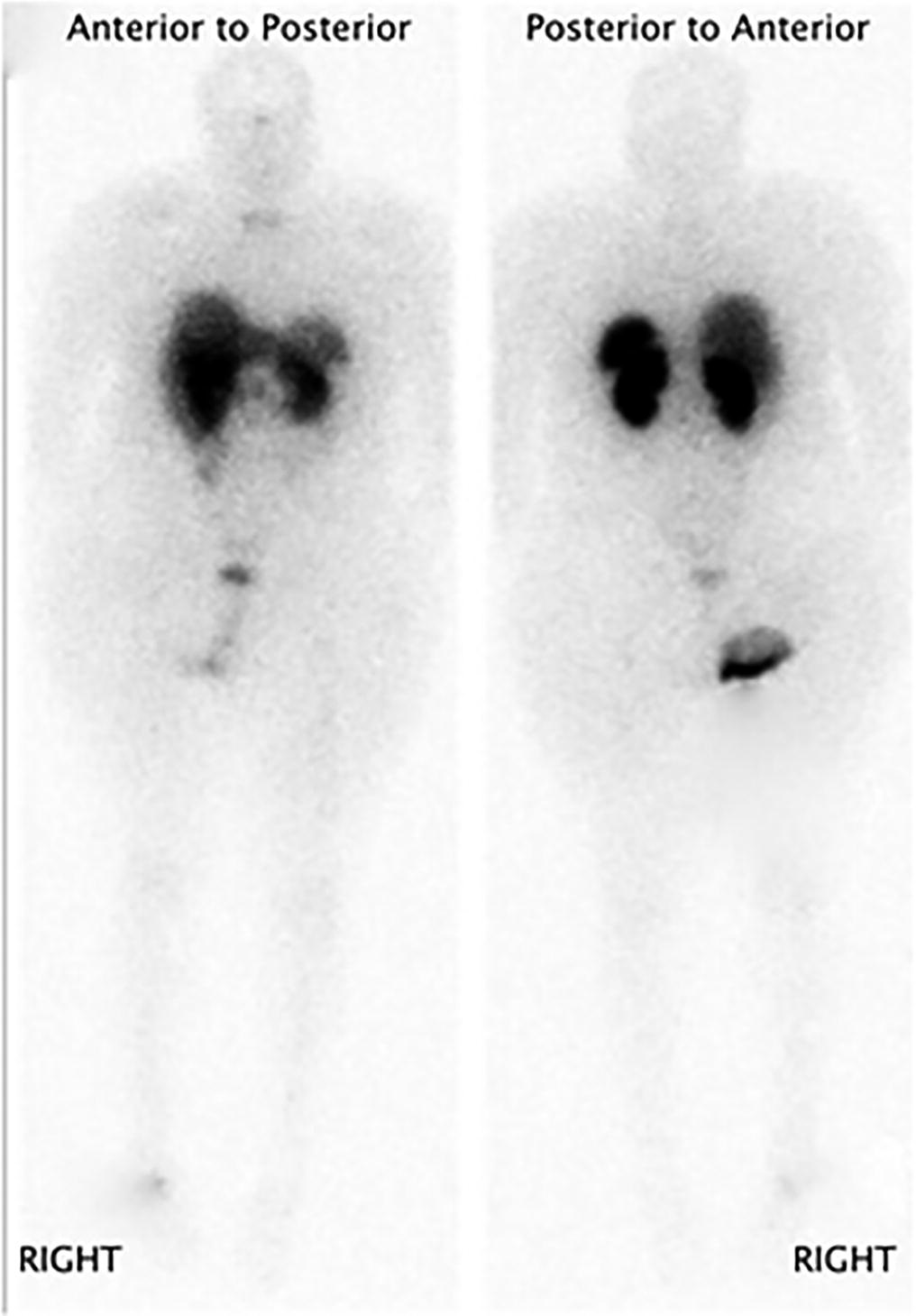
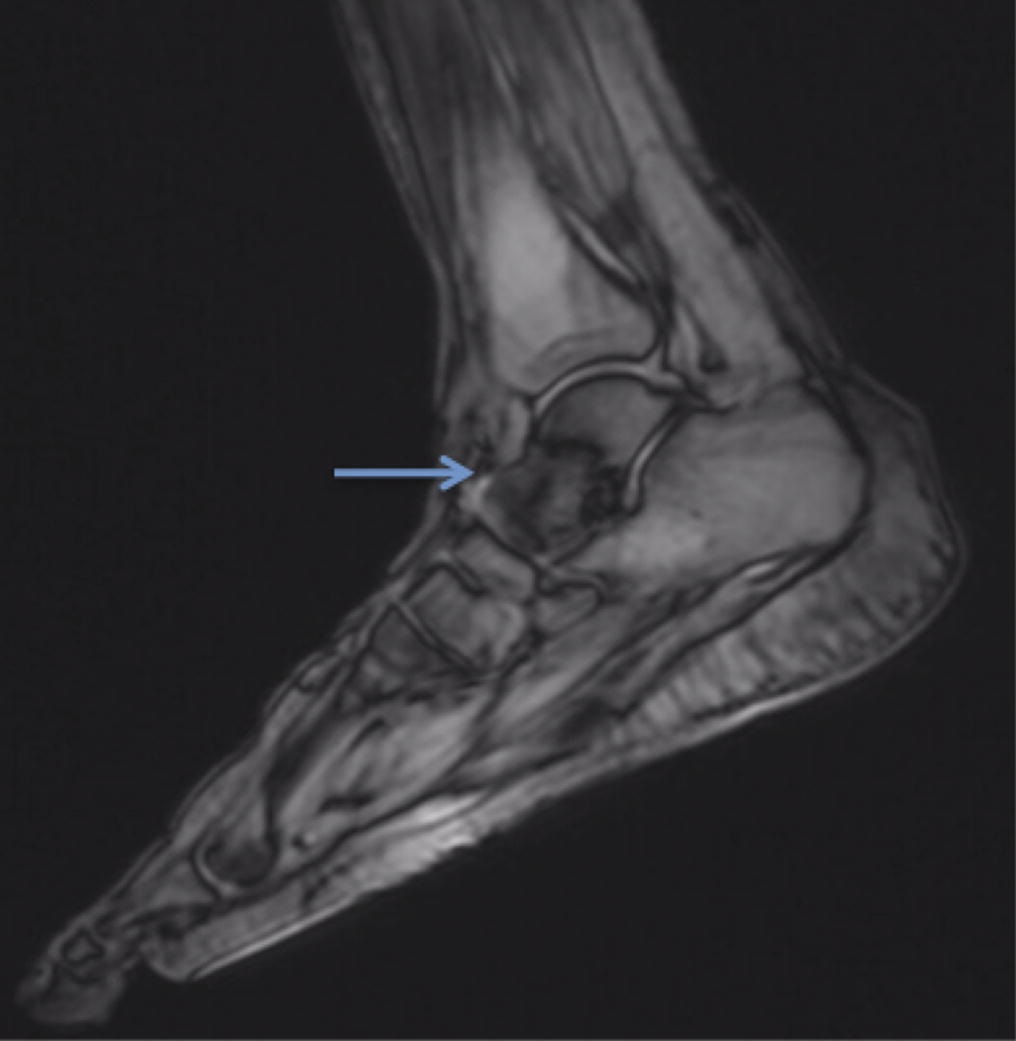
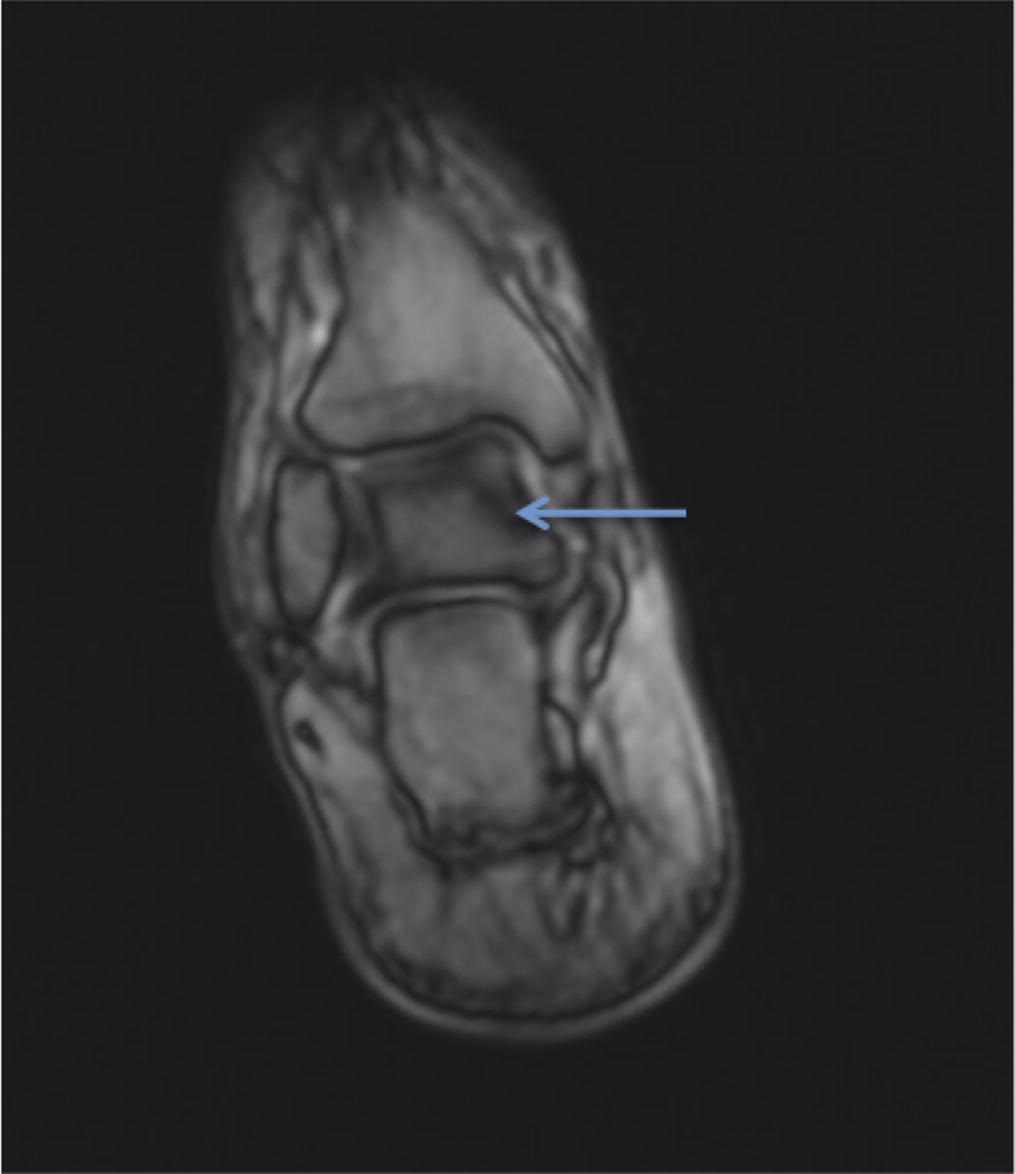
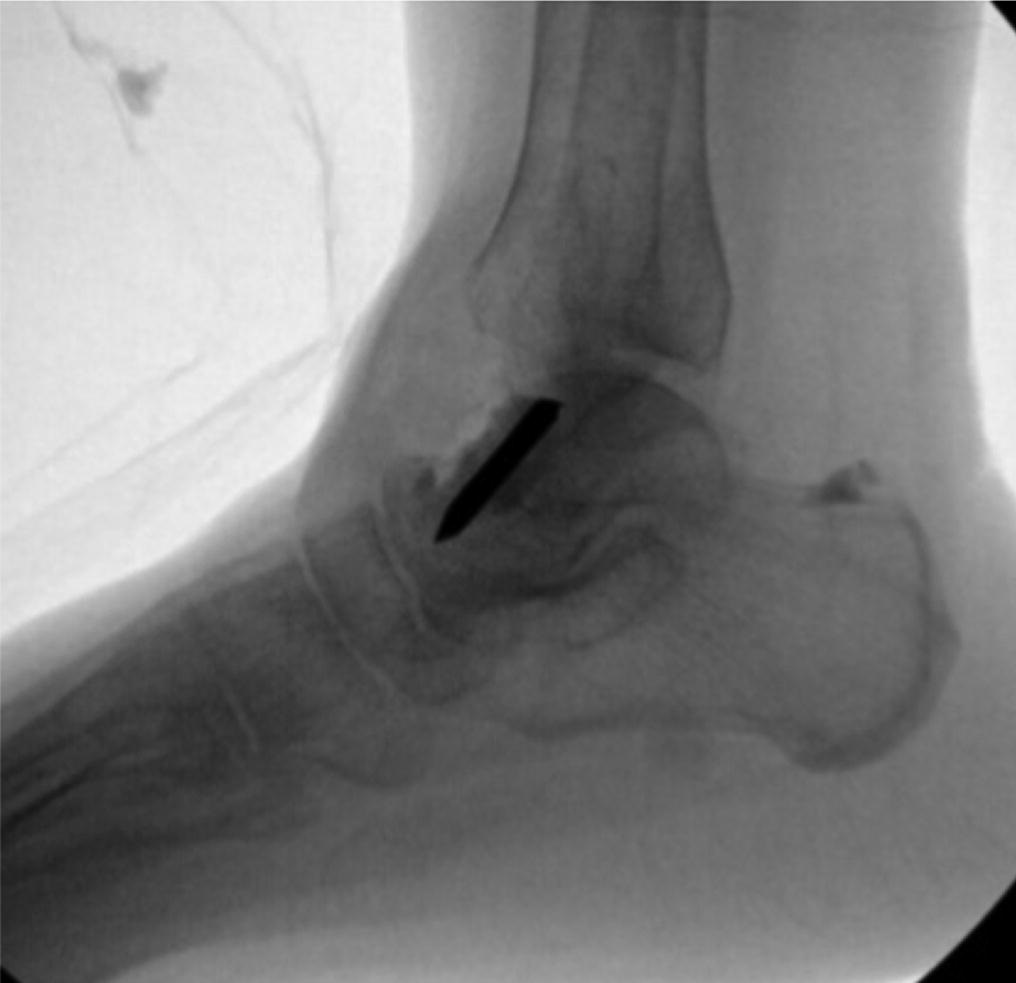
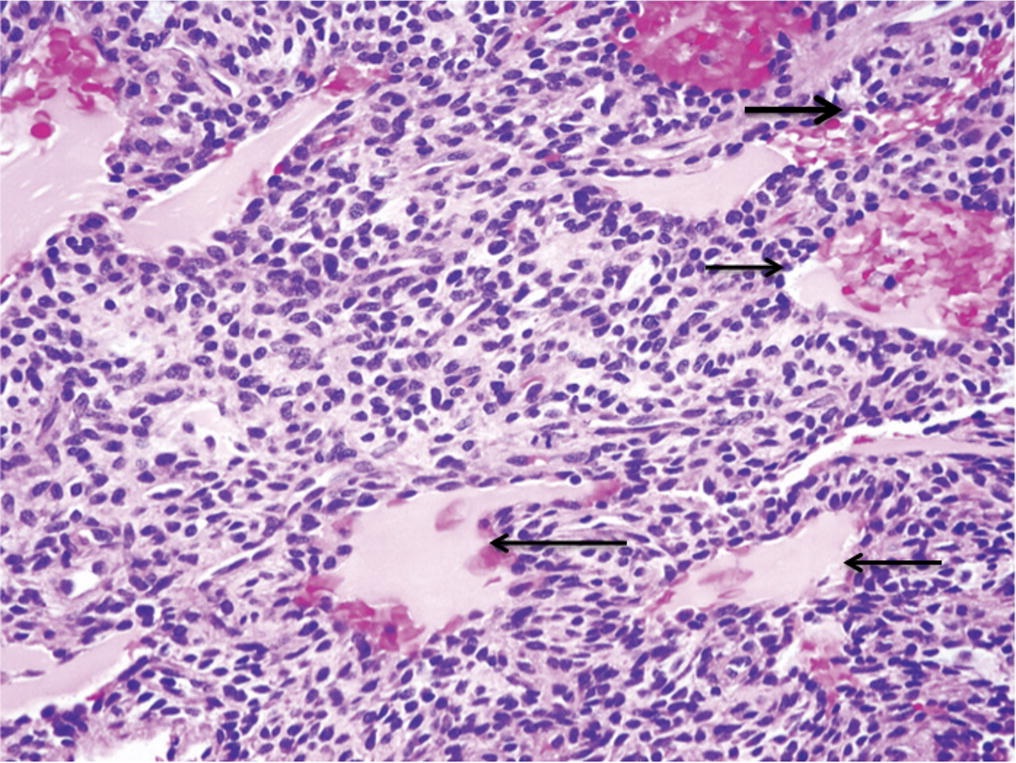
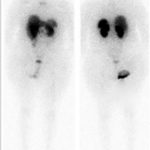 Fig. 1
Fig. 1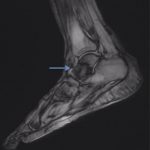 Fig. 2
Fig. 2 Fig. 3
Fig. 3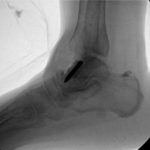 Fig. 4
Fig. 4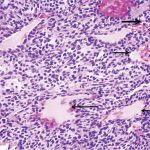 Fig. 5
Fig. 5