A 55-year-old man was referred to a musculoskeletal oncologist for a 4-year history of left lateral forefoot pain and swelling. The medical history included environmental and activity-related asthma and 2 knee arthroscopies for meniscal symptoms. He was a nonsmoker and worked as a teacher. The pain was described as 5 of 10—dull in nature and mildly related to weight-bearing. The patient recalled stubbing his toes multiple times over many years, but developed chronic swelling only during the previous year. He reported having no constitutional symptoms. He had not tried any medicine to relieve the pain and reported that the pain had not curtailed any normal activities.
On examination, there was fullness about the fourth and fifth toes, and he experienced tenderness to palpation along the distal aspect of the fifth metatarsal. There was an obvious firm, nonmobile, mildly tender mass between the metatarsals with no Tinel sign, thrills, or bruits. Sensation in the toes was normal. There was no lymphadenopathy proximally in the leg.
Radiographs revealed a mass extending along the medial side of the fifth metatarsal head and metatarsophalangeal (MTP) joint (Fig. 1-A). The mass was approximately 3 cm in size, with a sclerotic border and internal calcifications. There was evidence of erosion in the metatarsal head and neck. The mass (1.8 × 2.1 × 2.5 cm) was isointense to muscle on T1-weighted magnetic resonance imaging (MRI) (Fig. 1-B) and heterogeneously hyperintense on T2-weighted MRI (Fig. 1-C) with internal calcifications. There was a microfracture in the metatarsal neck, along with substantial adjacent osseous edema. The erosion seen on radiographs was confirmed with MRI. A technetium-99 (Tc-99m) bone scan showed mild uptake at the border of the lesion with no signal in the mass. Results of the serologic studies, including basic blood-cell count, chemistry, ESR (erythrocyte sedimentation rate), CRP (C-reactive protein), and uric acid, were all normal. After a thorough discussion, the patient agreed to undergo a biopsy.
A 2-cm longitudinal incision was made on the dorsum of the foot at the level of the distal metatarsal (Fig. 2-A). The mass appeared as a chalky white material, and several small pieces were taken for intraoperative analysis, including the use of polarized light microscopy (Fig. 2-D). The remainder of the lesion was excised, and the sclerotic border was curetted to induce healing. Postoperative pathology results are shown in Figs. 2-B and 2-C.
The results of the biopsy show crystal deposition associated with chondroid metaplasia of soft tissue. Evaluating the histology with polarized light demonstrates the rectangular and rhomboid yellow and blue crystals characteristic of calcium pyrophosphate crystals. The patient was diagnosed with tophaceous pseudogout (calcium pyrophosphate deposition disease).
The postoperative course was unremarkable. The patient was referred to a rheumatologist for work-up and possible systemic treatment, but declined additional treatment because the symptoms had been alleviated. Following 3 months of normal postoperative observation with no radiographic recurrence, the patient declined regular follow-up visits until 33 months later. He had not returned earlier because he had been completely pain-free and was functioning normally. After 33 months, he reported brief episodes of pain approximately once a week that lasted <30 seconds. He was not limited in any activities, and there was no visible mass or tenderness. Radiographs showed that a small amount of residual, calcified tophus, which had been observed postoperatively, had not changed, and approximately half of the eroded bone had filled in. The patient had no other symptoms.
Proceed to Discussion >>Reference: Krochak R, Culbertson MD, Vigorita V, Goodman H. Atypical tumoral presentation of calcium pyrophosphate deposition disease: a case report. JBJS Case Connect. 2016 Oct 26;6(4):e86.
Pseudogout is a member of the class of crystalline arthropathies caused by calcium pyrophosphate deposition in joints, collectively termed calcium pyrophosphate deposition disease (CPPD). Richette et al. classified CPPD into 3 subsets: chondrocalcinosis, pyrophosphate arthropathy, and pseudogout. Chondrocalcinosis is the calcification of articular fibrocartilage or hyaline cartilage as seen with radiography, pyrophosphate arthropathy is defined as structural abnormalities in cartilage and bone, and pseudogout manifests as acute synovitis. Other classification systems simply classify CPPD as either “common/diffuse,” which encompasses the above-mentioned syndromes, or as “tumoral/tophaceous,” which is a rare and more aggressive form, as was found in the current case.
The prevalence of CPPD primarily has been examined in terms of chondrocalcinosis; 1 community study in the United Kingdom reported a prevalence of 7%, while the Framingham survey, which looked at knee joints in patients over the age of 63 years, yielded an 8.1% occurrence. The prevalence increases with age, ranging from 3.7% in 55 to 59-year-old patients to 17.5% in 80 to 84-year-old patients. Other studies that examined osteoarthritic joints via synovial fluid samples also demonstrated a high prevalence of calcium pyrophosphate crystals, with rates between 25% and 43%. While chondrocalcinosis is asymptomatic, pseudogout is an acute, crystal-induced synovitis. Like classic gout, the attacks are usually self-limiting; can be monoarticular or oligoarticular; and are characterized by pain, erythema, warmth, and swelling of the affected joint. Symptoms have been described as appearing suddenly and lasting for about 10 days, with cluster attacks lasting several weeks.
Tumoral CPPD is a rare clinical manifestation and has been described as primarily affecting the temporomandibular joint, the knee, and the hand, with a few isolated cases in the ankle, the wrist, and the cervical spine. The metatarsals and the metacarpals are rarely involved. Tophaceous CPPD appears as calcified lesions with a granular or fluffy pattern, with adjacent bone showing pressure erosion. A few cases have been described around the first MTP joint; however, each had a feature that pointed to a more acute inflammatory etiology, such as gout, which is more common in this location. Examples include a flare-up of pain and inflammation, suggesting an acute gouty arthritis, or severe pain and functional weakness of only a short duration, accompanied by swelling and erythema. Furthermore, calcium pyrophosphate and monosodium urate crystals have been found together in several joints.
The present case demonstrates an example of tumoral/tophaceous CPPD in an unusual location, and includes severe erosion of the adjacent metatarsal bone. This case is unique in that there was no specific instigating event, injury, or synovitic episode. The pain was chronic in nature, with attacks increasing in severity and frequency over several years and with symptoms eventually becoming constant in nature.
Several factors have been identified for the development of CPPD-related syndromes, including age, osteoarthritis, and metabolic diseases. Age appears to be the greatest risk factor for sporadic disease with asymptomatic chondrocalcinosis. There is also a well-recognized connection to osteoarthritis; however, a causal relationship between the pro-inflammatory crystals and joint degeneration is not yet clear. Studies of synovial fluid collected during joint replacement surgeries and from knees following meniscectomy have also correlated prior joint damage with CPPD deposition. Some metabolic diseases have been associated with CPPD deposition, including hemochromatosis, hyperparathyroidism, hypomagnesemia, and hypophosphatasia.
Several genetic loci have been described as potential causes for familial chondrocalcinosis, leading to earlier onset and increased severity of joint damage. Mutations in ANKH (ankylosis human), a gene on the short arm of chromosome 5, have also been linked to chondrocalcinosis 2.
As opposed to common forms of CPPD, radiographic diagnosis for the tumoral form of CPPD is problematic. The diaphanous depositions and often insidious onset mimic the presentation of several bone tumors. Fluffy calcium deposits, marginal calcification, and osseous erosion are often seen with tumoral pathologies. Diagnosis by fine-needle aspiration and findings of positively birefringent rhomboid crystals have been reported in several studies, and may be useful as a precursor to biopsy. This technique has been used successfully in a soft-tissue mass of the neck, lumbar vertebral bodies, and various joints throughout the body. Finally, histologic confirmation is made with short rhomboid weakly positively birefringent crystals, seen in a mixed cellular background with foreign-body-type giant cells, a possible granulomatous response, and metaplastic chondroid tissue. As seen in this patient, chondroid metaplasia is often the dominant feature on histology (Figs. 2-C and 2-D) and is responsible for the bright T2-weighted fluid signal seen on MRI.
This case was unique in that the initial presentation was highly suspicious for a neoplastic pathology, with imaging and clinical signs suggesting a slow-progressing malignancy. Biopsy and curettage definitively proved that the lesion was an atypical form of pseudogout. Clinicians who encounter cases exhibiting gout-like clinical signs combined with radiographic evidence suggestive of tumoral bone disease should consider tophaceous pseudogout in the differential diagnosis and include analysis with polarized light microscopy when examining biopsy specimens.
Reference: Krochak R, Culbertson MD, Vigorita V, Goodman H. Atypical tumoral presentation of calcium pyrophosphate deposition disease: a case report. JBJS Case Connect. 2016 Oct 26;6(4):e86.
What is the diagnosis?
Tophaceous pseudogout (calcium pyrophosphate deposition disease)
Tophaceous gout (uric acid crystal deposition)
Both gout and pseudogout
Osteochondroma
Bizarre parosteal osteochondromatous proliferation

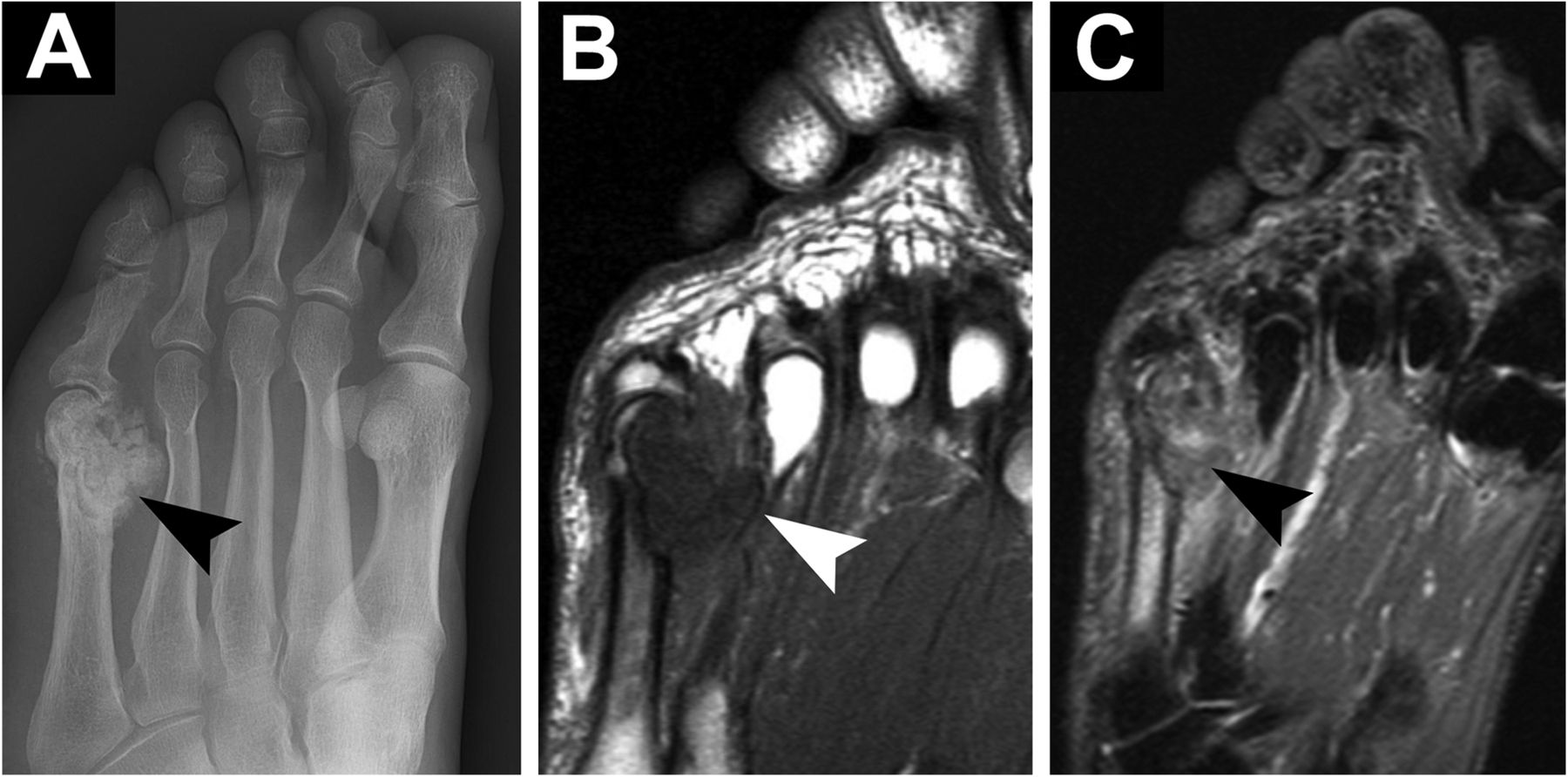

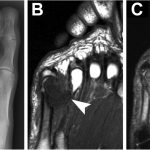 Fig. 1
Fig. 1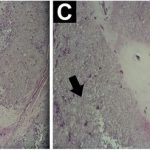 Fig. 2
Fig. 2