A thirty-five-year-old man presented with incapacitating back pain radiating down the posterior aspect of both legs. Magnetic resonance imaging (MRI) demonstrated progressive spondylosis and multilevel foraminal stenosis. He had undergone two previous limited posterior decompressions without lasting improvement. Refractory to nonoperative care, the patient underwent anterior lumbar interbody fusion (ALIF) of L3-L4, L4-L5, and L5-S1 with polyetheretherketone (PEEK) interbody cages. One large INFUSE kit (Medtronic) was divided among the three levels, and one-third of the kit (two sponges) was used at each level (approximately 4 mg per interspace). Prior to placing the sponges inside each cage, milled cancellous allograft bone was wrapped inside each of the INFUSE sponges. Additional milled allograft bone without INFUSE was placed surrounding the cage at L5-S1. The patient underwent L3-S1 pedicle screw instrumentation and fusion with use of a minimal access surgical technique in which the facet joints were decorticated and allograft was placed prior to screw placement (Figs. 1-A and 1-B). No INFUSE was used posteriorly. The patient had an uncomplicated hospital course and was discharged home on postoperative day four. He was then seen at regularly scheduled follow-up intervals at six weeks, three months, six months, nine months, and one year; no new symptoms were present.
Approximately one year after what appeared to be a successful spinal fusion, the patient presented to his primary care provider with what he perceived to be a new and unrelated symptom of abdominal pain, described as “something sticking him in his lower abdomen.” Computed tomography (CT) of the abdomen demonstrated a radiodense curvilinear abnormality that extended anterior from the psoas to the left pelvic brim (Fig. 2). The mass was excised, and there was an intraoperative finding of a “bony branching ossified structure” within the retroperitoneum, extending to the anterior abdominal wall. Histological examination of the tissue revealed “fragments of benign bone, dense collagen, and an acellular material.” The postoperative course was uneventful with resolution of the abdominal symptoms.
The patient was diagnosed with bone morphogenetic protein-induced heterotopic bone.
Proceed to Discussion >>Reference: Steeby SF, Jeffries JT, Choma TJ, Kuhns CA. Formation of abdominal heterotopic bone following the utilization of bone morphogenetic protein in anterior lumbar spinal fusion: a case report. JBJS Case Connect. 2014 Jan 08;4(1):e5.
In 2008, more than 400,000 spinal fusions were performed in the United States. The literature suggests that successfully achieving arthrodesis improves the outcome of fusion surgery. Recombinant human bone morphogenetic protein (rhBMP-2) has been employed to facilitate fusion with the assumption that it is safe and effective. As its usage has grown, there has been a parallel increase in complication reports, including heterotopic bone formation. Generally, heterotopic bone formation has been noted in close proximity to the location of implantation or has been associated with the administration of an exceptionally high dose of rhBMP-2.
In this case report, we describe a complication that occurred even though the rhBMP-2 dose was consistent with the manufacturer’s recommendation (although admittedly off-label) and the collagen sponge carrier was confined to the central aspect of the implants. Despite these conditions, heterotopic bone developed in the retroperitoneum along the entire dissection path. It is still unknown to us why this particular patient developed abdominal heterotopic ossification. The primary surgeon has used rhBMP-2 routinely in ALIFs without any known prior incidence of abdominal heterotopic bone formation. Two potential explanations for this must be considered. First, the retroperitoneal space may have been contaminated with the rhBMP-2 during insertion of the anterior implants. However, this seems unlikely because the BMP was added to the collagen sponges and placed inside the cages on the back table and then carefully inserted into the disc spaces without contact with the surrounding soft tissues.
The second possibility is that postoperative hematoma or seroma fluid bathed the disc spaces, allowing some elution of the rhBMP-2 along the exposed retroperitoneal space, inducing the heterotopic bone formation. Dragoo et al. demonstrated abundant bone formation in mice that were injected with multipotent progenitor cells found in human adipose tissue that had been transfected with an adenovirus carrying the gene for rhBMP-2. In our patient, it is conceivable that exposure of adipose tissue (the retroperitoneal fat) to rhBMP-2 could have led to the development of ectopic bone formation.
Regardless of the mechanism, this case presents a concerning issue regarding the use of rhBMP-2 and the risk of bone formation distant from the carrier with use of only a modest dose of INFUSE. There may exist certain host factors yet to be characterized that are more likely to produce heterotopic bone formation when INFUSE is used. Ectopic bone formation might not be directly proportional to the dosage of BMP used or a result of the INFUSE sponge being contained in the cage.
Interestingly, Kim et al. described a similar case in which a patient developed heterotopic bone formation in the pelvis following use of BMP-7 in combined anterior and posterior spinal fusion. It may be that the use of osteoinductive materials in general carries a risk of distal heterotopic bone formation, which requires further understanding.
Arguments have been made that while BMP may increase the likelihood of ectopic bone formation, it rarely leads to any adverse event or clinical symptoms. While cases of soft-tissue bone formation may go unnoted in several circumstances, some patients do experience clinical manifestations of the side effects of BMP. In our case, the patient needed a second operation, an expensive and time-consuming endeavor that put him at risk.
A recent meta-analysis of eighteen studies from seven countries reported that, at two to four years of follow-up, patients whose fusion procedure included the use of rhBMP-2 did not demonstrate the statistically significant fusion rates shown by those that did not use rhBMP-2. The described complication in this report, coupled with others in the literature, and an unclear improvement in outcomes present cause for surgeons to carefully consider the routine use of rhBMP-2 as an adjuvant to lumbar fusion surgery.
Reference: Steeby SF, Jeffries JT, Choma TJ, Kuhns CA. Formation of abdominal heterotopic bone following the utilization of bone morphogenetic protein in anterior lumbar spinal fusion: a case report. JBJS Case Connect. 2014 Jan 08;4(1):e5.
What is the diagnosis?
Intra-abdominal myositis ossificans
Heterotopic mesenteric ossification
Partially calcified retained surgical sponge
Ossified prosthetic mesh
Bone morphogenetic protein-induced heterotopic bone

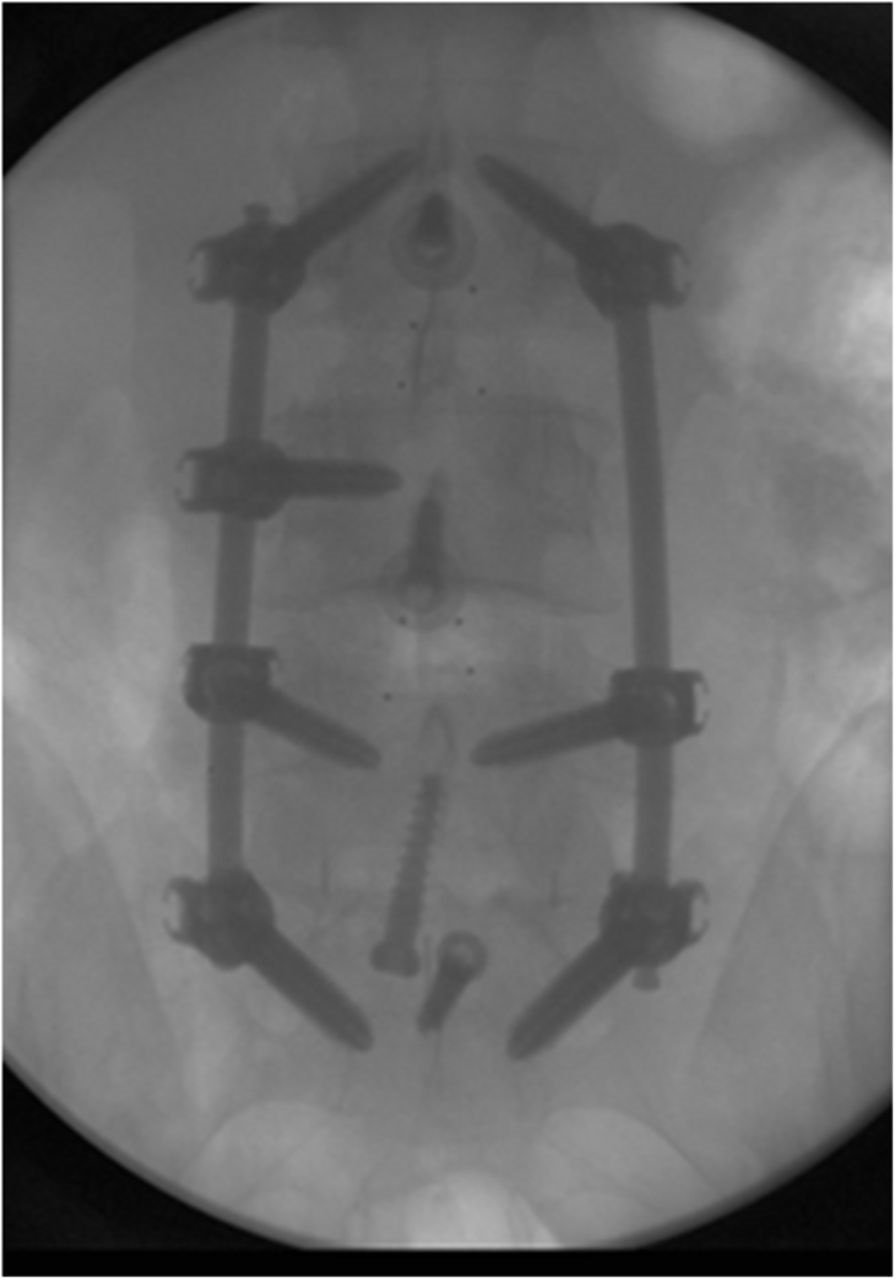

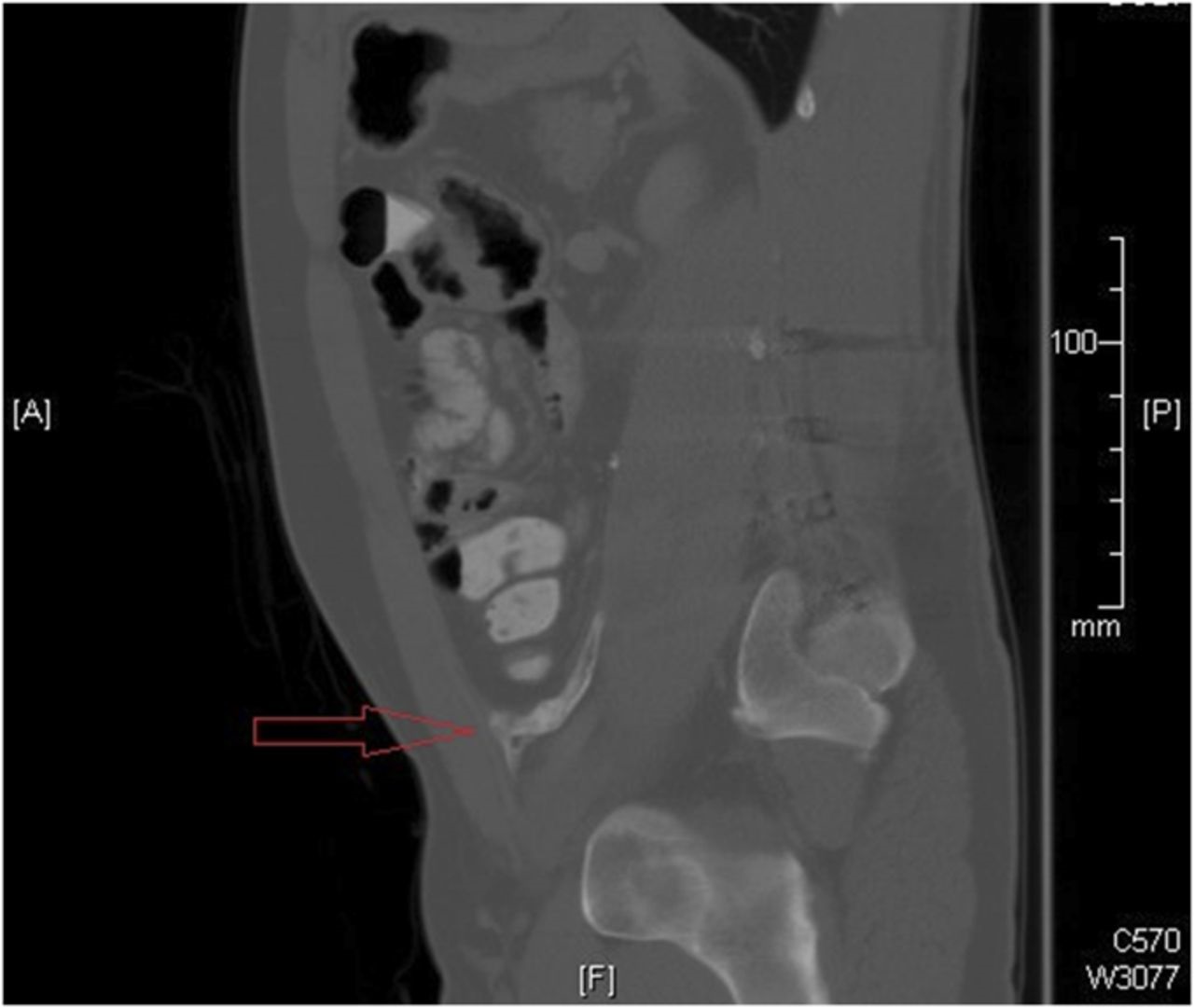
 Fig. 1-A
Fig. 1-A Fig. 1-B
Fig. 1-B Fig. 2
Fig. 2