A 14-year-old boy presented for evaluation of adolescent idiopathic scoliosis. He initially had been seen 3 years prior; at that time, a dextrothoracic curvature of 20° had been noted. When he had presented after a 2-year interval, severe advancement of the deformity had been observed.
The patient noted no pain or neurologic deficits. He reported an active lifestyle, including full-contact sports. On physical examination, a substantial spinal deformity was evident, with a right thoracic paraspinal prominence, a low left thoracic prominence, and a low thoracic gibbus deformity. On standing radiographs, the spine demonstrated a high dextrothoracic curvature of 35° and a low levothoracic curvature of 45°. Also evident was partial absence of the left ribs at T9 and T10 (Fig. 1). On the lateral radiograph, there was a sharp kyphotic thoracic curvature of 61°.
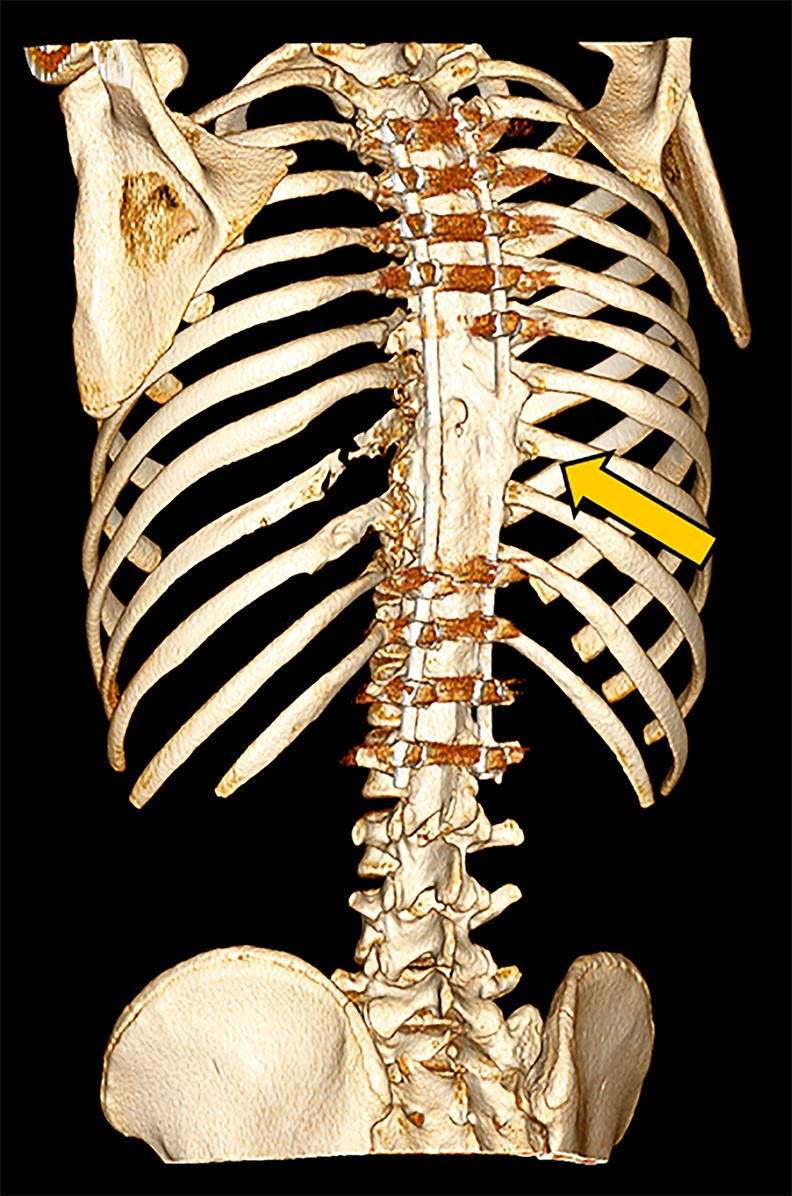
Magnetic resonance imaging (MRI) and computed tomography (CT) were performed for further characterization and diagnosis. MRI showed multiple areas of syrinx in the cord from C6 to T12. Hyperintense signals on T1 and T2-weighted images were seen in the vertebral bodies. These signals were contrast-enhancing and fat-suppressible, consistent with vertebral involvement by lytic process (Fig. 2-A). Three-dimensional (3D) CT reconstruction demonstrated loss of the left ribs at T9 and T10 and the posterior elements involving the T6 spinous process, the C7 spinous process, the T8 transverse and spinous processes, the T9 left transverse and spinous processes, the T10 transverse and spinous processes, and the T11 spinous process (Fig. 2-B). The loss of posterior elements threatened impending structural compromise and spinal cord compression.
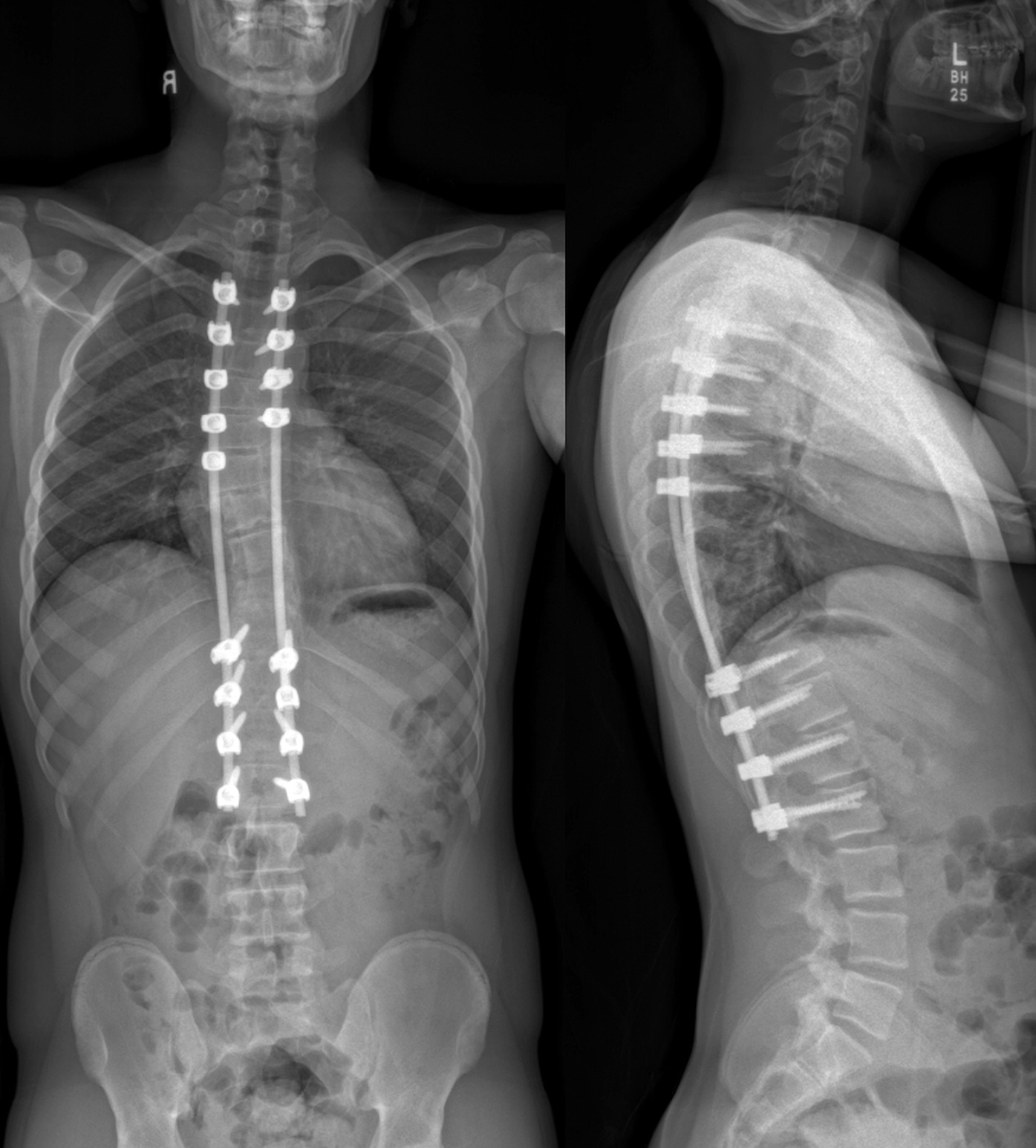
The family was counseled in detail regarding the treatment options. A sequential course of medical and surgical treatment was elected. The patient began oral therapy with sirolimus 7 weeks prior to surgical correction of the kyphotic deformity. The 7-week duration was chosen because of the time needed for operative planning and coordinating of the surgical schedule. The dosing strategy was 0.8 mg/m2 twice daily, titrated to a 12-hour trough of 7 to 13 ng/mL. Sirolimus was held 2 days preoperatively, and the patient underwent posterior instrumented spinal fusion from T3 to L2 with facetectomies (Fig. 3). Extensive bone loss over the posterior elements was present with exposed dura. Sections of tissue excised during the procedure are shown in Figures 4-A, 4-B and 4-C.
The patient was diagnosed with Gorham-Stout disease (GSD). The excised specimen demonstrated enlarged medullary lymphatic channels highlighted by D2-40 immunostaining and thin bone cortex, consistent with the diagnosis of GSD (Figs. 4-A, 4-B, and 4-C). D2-40 is a marker specific to lymphatic endothelial tissue, which is not typically found in osseous tissue. It is not expressed in arterial endothelium, which helps to distinguish GSD from hemangiomas.
The patient was transferred to the intensive care unit for monitoring. Sirolimus therapy was reinitiated 2 days postoperatively, and the patient was discharged without incident after meeting inpatient and physical therapy milestones. The postoperative course was notable for the absence of wound problems or chylothorax.
Bisphosphonate therapy (zoledronic acid, 0.5 mg/kg/dose) was initiated 3 months postoperatively; it was given intravenously on an alternating-month schedule. Routine follow-up visits were uneventful. At the 18-month follow-up, radiographs (Fig. 5) showed intact hardware and maintained alignment. At the 24-month follow-up, the patient remained on sirolimus and bisphosphonate therapy. By this point, he had returned to full activity, participating in crew and soccer without limitation. CT demonstrated successful fusion of the posterior aspect of the spine as well as partial restoration of the ribs at T9 and T10 (Figs. 6-A and 6-B).
Proceed to Discussion >>Reference: Mo AZ, Trenor CC, Hedequist DJ. Sirolimus therapy as perioperative treatment of Gorham-Stout disease in the thoracic spine. A case report. JBJS Case Connect. 2018 Sep;8(3):e70
Although the exact pathophysiology of GSD is unclear, the association between lymphatic proliferation and progressive osteolysis is well-established. Gorham and Stout originally postulated that the presence of angiomatosis resulted in an active hyperemia, disrupting bone metabolism and shifting the balance to favor osteoclastic activity. Spinal involvement can lead to instability, neurologic compromise, spinal cord transection, or chylothorax. Disease of the ribs, the scapula, or the thoracic vertebrae may involve direct extension of lymphatic fluid into the pleural cavity. Treatment of spinal GSD requires close observation and multidisciplinary clinical decision-making.
Because there is no standard disease presentation, the management of patients with GSD is case-specific. Available treatment modalities include chemotherapy, radiation therapy, and surgical resection. Predominant medical therapies to date include bisphosphonate or interferon therapy. Sirolimus, an mTOR (mechanistic target of rapamycin) inhibitor, is increasingly used in lymphatic diseases, and is a promising new option for GSD treatment. mTOR is a master switch in cellular pathways that regulate cell growth and proliferation, and upregulation can lead to increased angiogenesis and lymphangiogenesis. Inhibition of mTOR has antitumoral and antiangiogenic effects in lympangitic disorders, including GSD.
Between 1947 and 2016, there were 59 spine cases of GSD reported in the literature. Of these 59 cases, 49% involved the cervical spine, and 46% involved the thoracic spine. Twenty-eight underwent surgical correction; 18 required revisions due to resorption of grafted bone, which is a substantial risk that can require multiple revisions. Tateda et al. advocated for sequential chemotherapy (interferon alpha-2B) followed by surgical resection. Medical optimization of patients with GSD through neoadjuvant targeting of lymphangitic tissue offers a means to enhance surgical outcomes.
Sirolimus has been used successfully to treat lymphangitic diseases such as kaposiform lymphangiomatosis, lymphatic malformation, diffuse lymphangiomatosis, and GSD. The risk of intraoperative chyle drainage poses a risk for increased complications and difficulty with surgical dissection. Postoperative drainage poses a risk for wound complications and the development of chylothorax.
Diseases involving angiogenesis and lymphangiogenesis are marked by increased activation of the phosphatidylinositol 3-kinase (PI3K)/Akt pathway and subsequent enhanced expression of vascular endothelial growth factor. This pathway is critical to cell growth and survival. Inappropriate activation has been shown to result in tissue overgrowth in association with vascular anomalies. Sirolimus can halt bone loss and control the underlying disease in patients with GSD. Clinical trials of sirolimus in patients with tuberous sclerosis or lymphangioleiomyomatosis involving the PI3K/AKT pathway have been promising. One phase-II clinical trial of sirolimus in 61 patients with lymphatic malformations and vascular anomalies included 3 patients with GSD; 53 of 57 patients (including the 3 patients with GSD) who were eligible for evaluation had a response to therapy within 6 courses of treatment. Adverse effects of sirolimus therapy in these patients included mouth and lip ulcers, diarrhea, abdominal pain, nausea, sore throat, headache, dizziness, elevated cholesterol, and leg swelling.
In summary, given the high morbidity in GSD with spinal involvement, combined medical and surgical therapy was recommended for our patient. Medical management with sirolimus allows for the potential to control lymphatic lesions prior to surgical intervention and stabilization. Complementary postoperative bisphosphonate therapy was used in conjunction with sirolimus therapy to help decrease bone loss. Control of the lesions also offers the theoretical benefit of reducing the resorption of grafted bone, allowing for fusion and integration. While the necessary length of medical therapy is unclear in patients with GSD, this case demonstrates a rapid response to sirolimus therapy within 7 weeks of surgery.
Reference: Mo AZ, Trenor CC, Hedequist DJ. Sirolimus therapy as perioperative treatment of Gorham-Stout disease in the thoracic spine. A case report. JBJS Case Connect. 2018 Sep;8(3):e70
What is the diagnosis?
Multifocal hemangiomatosis
Angiosarcoma
Gorham-Stout disease
Aneurysmal bone cyst
Telangiectatic osteosarcoma

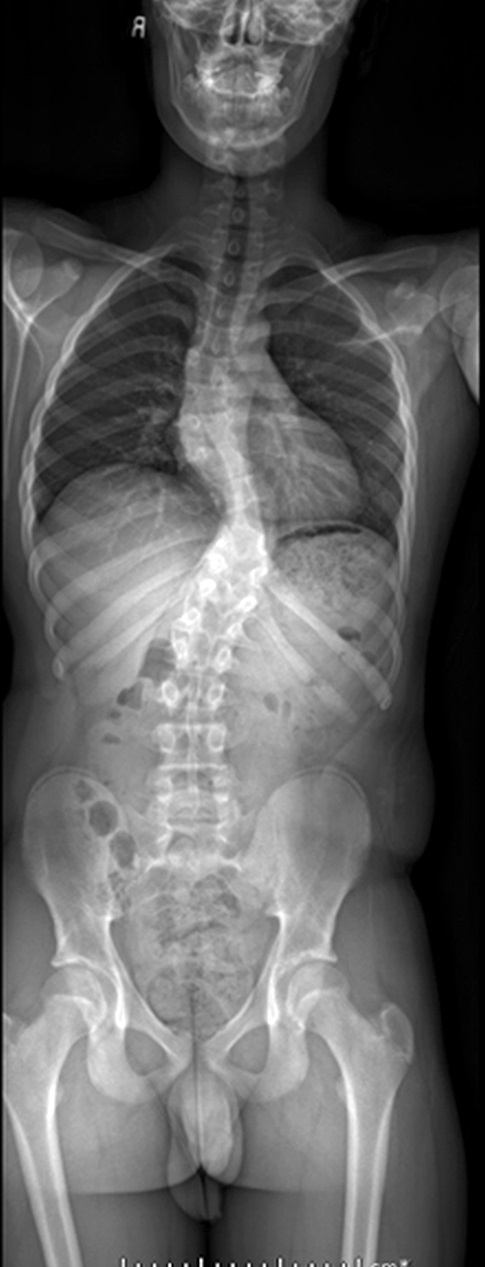

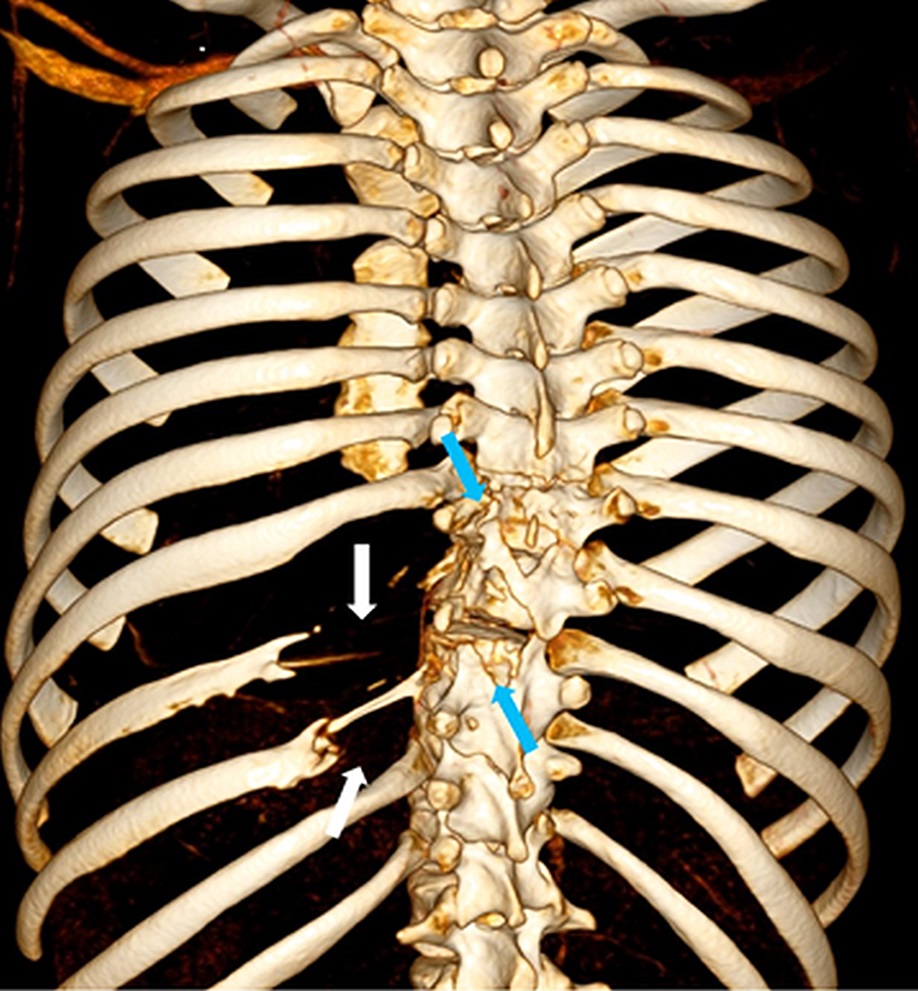
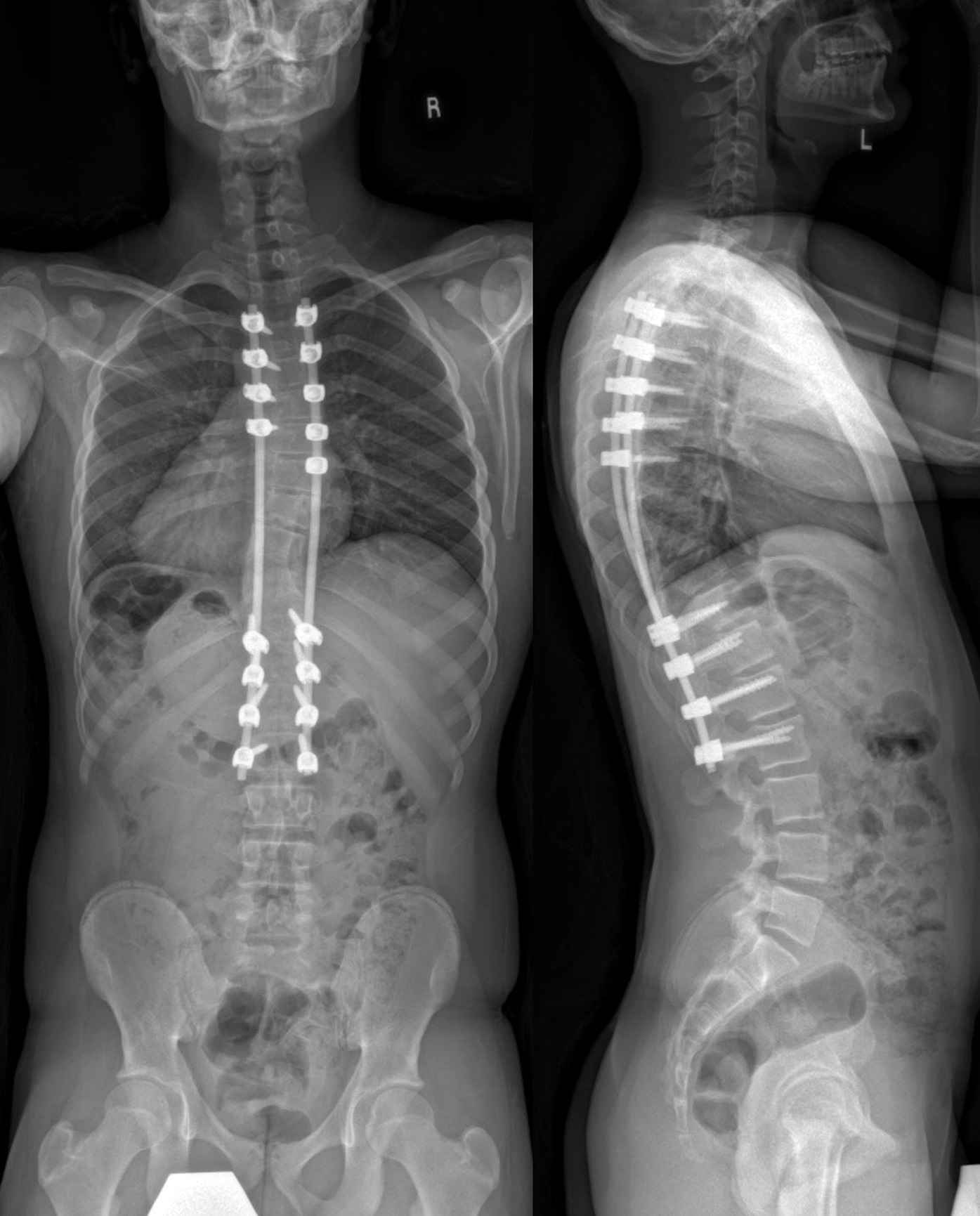

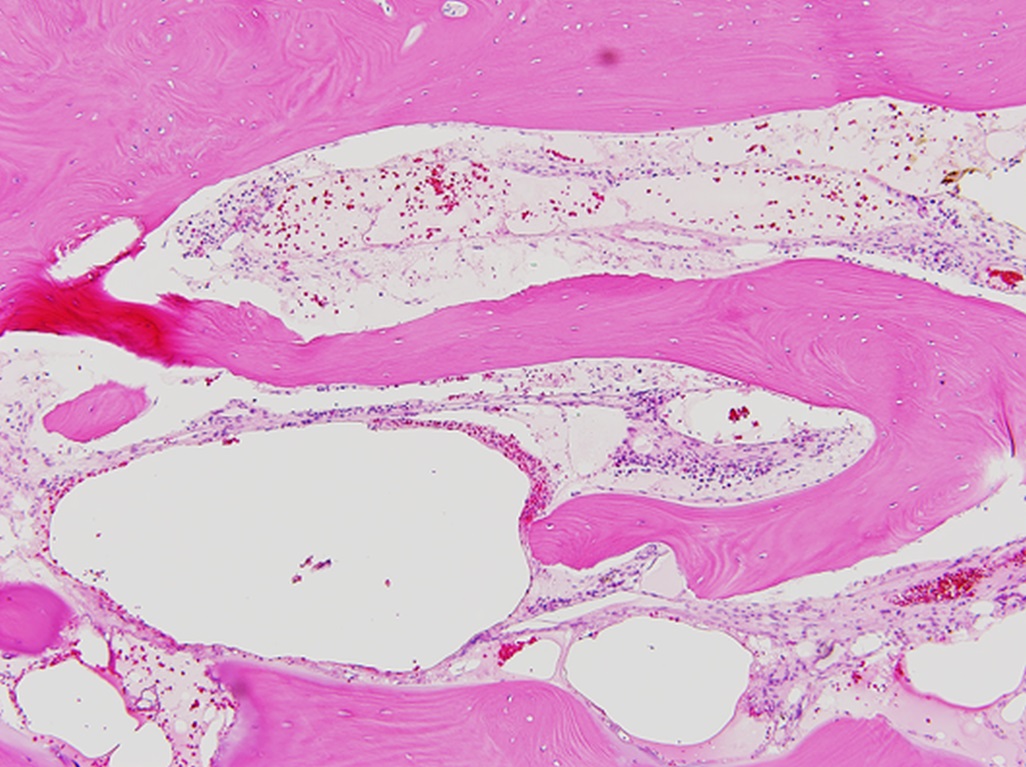
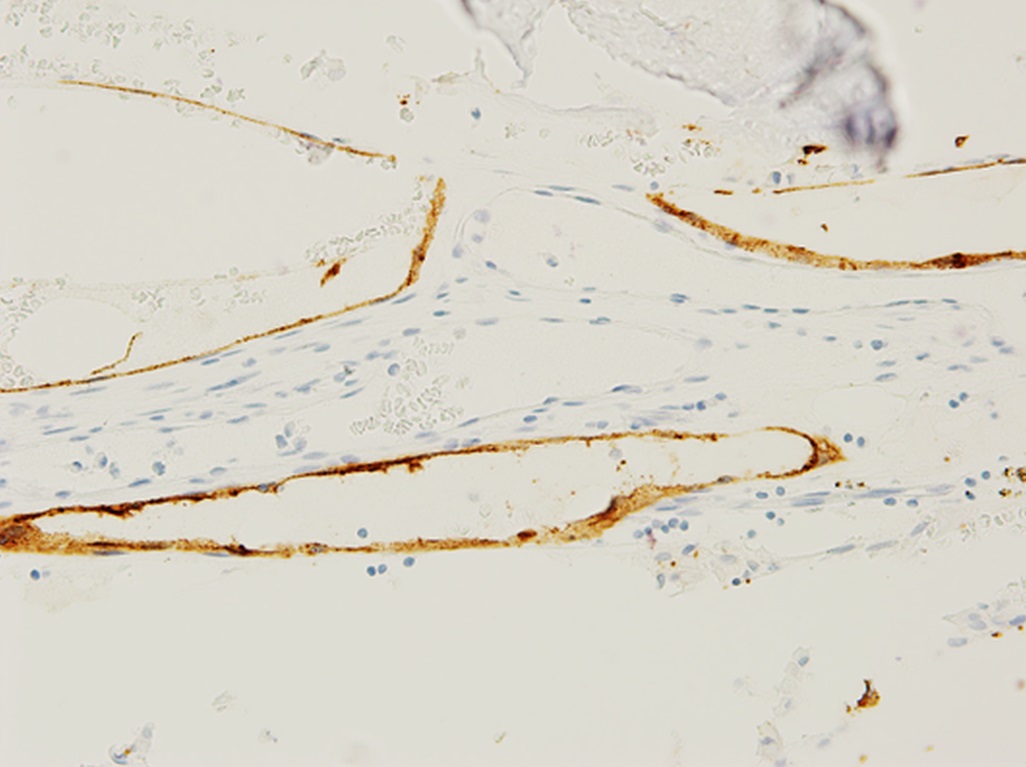
 Fig. 1
Fig. 1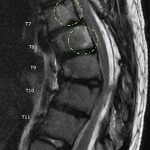 Fig. 2-A
Fig. 2-A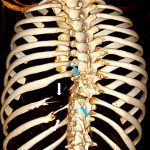 Fig. 2-B
Fig. 2-B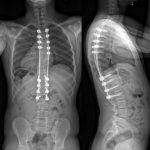 Fig. 3
Fig. 3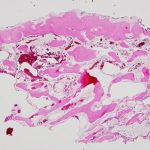 Fig. 4-A
Fig. 4-A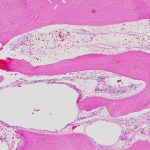 Fig. 4-B
Fig. 4-B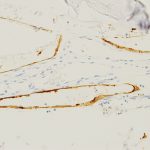 Fig. 4-C
Fig. 4-C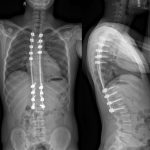 Fig. 5
Fig. 5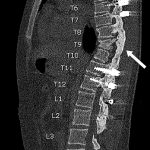 Fig. 6-A
Fig. 6-A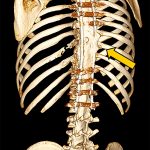 Fig. 6-B
Fig. 6-B