A 21-Year-Old Man with Persistent Worsening Calf Pain
January 5, 2022
A 21-year-old healthy man presented with a 1-year history of persistent right plantar fasciitis despite nonoperative treatment for over a year. On physical examination, he was experiencing pain over the plantar medial heel and had a gastrocnemius contracture based on the Silfverskiöld test with maximum dorsiflexion of 0° with the knee extended and 20° with the knee flexed. The patient elected to undergo a gastrocnemius recession.
In the operating room, a thigh tourniquet was used, and the right lower extremity was prepped with chlorhexidine gluconate 2% wt/vol and isopropyl alcohol 70% vol/vol and was sterilely draped. A 4-cm incision was made over the posterior medial lower limb at the gastrocsoleus junction, and a standard Strayer procedure was performed. Adequate release was evident with the ankle obtaining 15° of dorsiflexion with the knee extended. The wound was irrigated with normal saline, the deep layer was closed with Vicryl, and the skin was closed with nylon sutures. Xeroform and sterile 4 × 4 bandages were applied over the wound, and the patient was placed in a plaster posterior splint and was instructed to be non-weight-bearing for 2 weeks.
The patient returned to the office on postoperative day 10 with reports of subjective fever and calf pain. He had no objective temperature on office assessment. His splint was removed, and mild erythema was noted around the wound. The skin edges were darkened, but this was thought to be due to the blue surgical marking pen. The patient was placed back in a splint, was given a 7-day oral cephalexin course, and was scheduled to return in 3 days for a wound check.
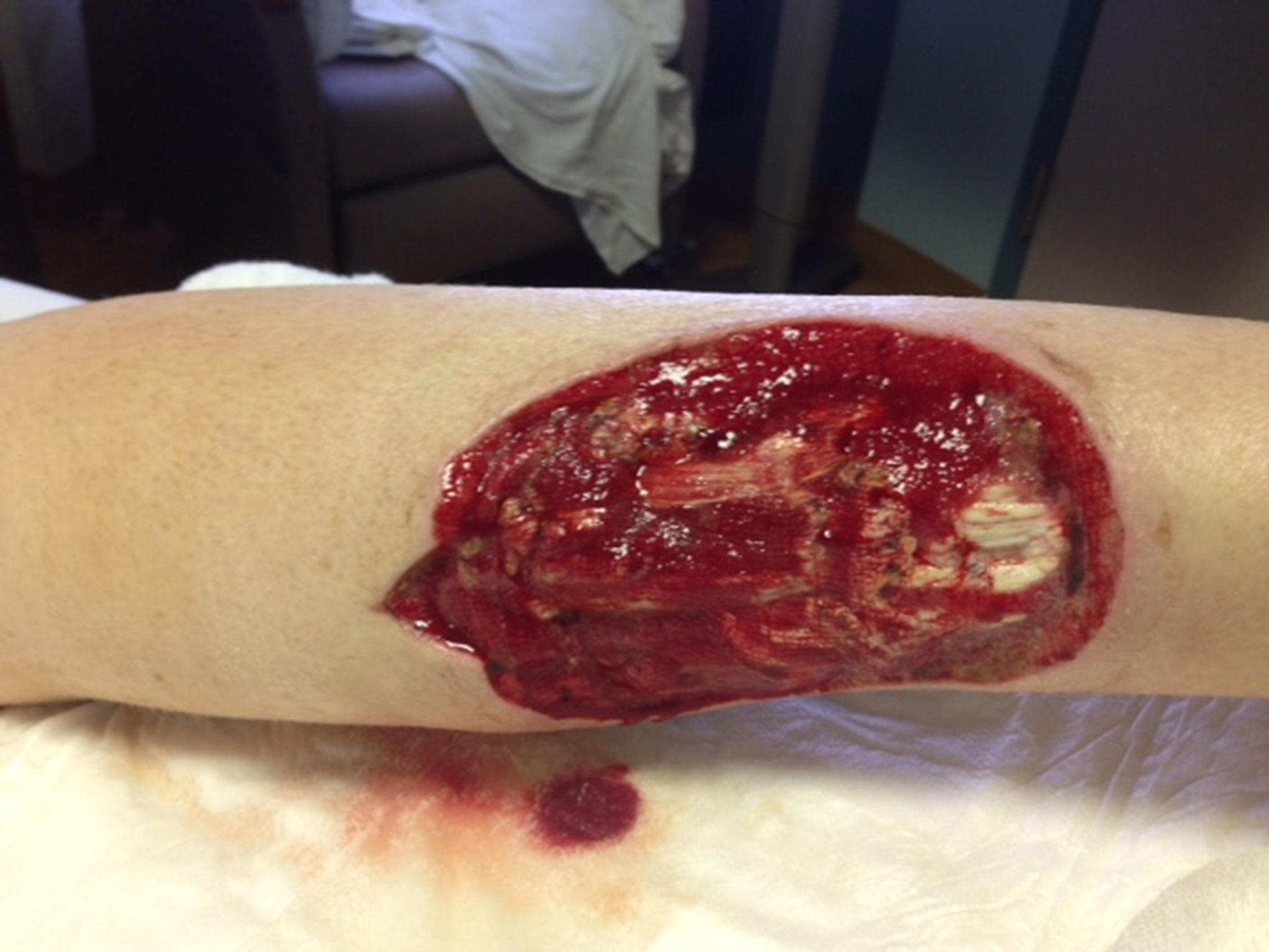
Two days later, the patient presented to the emergency department with increasing calf pain, and the area of darkness had expanded beyond the sutured edges (Fig. 1). Radiographs of the lower limb were normal, and there was no gas in the tissues based on the radiographs and the clinical examination for crepitus. He was afebrile and had a white blood cell count of 17,000/μL. Given the severity of the presentation of the wound despite clear etiology, the patient was taken to the operating room for surgical debridement and wound vacuum placement and was administered intravenous vancomycin and piperacillin with tazobactam. The area of necrosis surrounding the surgical wound was debrided, and there was no gross purulence, but the wound was soupy with brown discharge. Tissue samples were sent for Gram stain and culture and to the pathology department for microscopic evaluation.
The histology of the debrided tissue is shown in Figures 2-A and 2-B.
The histology was interpreted as showing nonseptated, ribbon-like hyphae with right-angle branching, consistent with mucormycosis. The patient was diagnosed with fungal hyphae consistent with mucormycosis.
Two days after the surgical procedure, the patient returned to the operating room after the area of erythema on the right calf had expanded and the brown necrotic edges of the wound had worsened. His wound was again thoroughly debrided to clean edges measuring 7 × 13 cm2, and a wound vacuum was replaced. The following day, 3 days after the initial debridement, the erythema had resolved around the edges.
Pharmacotherapy was changed to liposomal amphotericin B, and antibiotics were discontinued. A third surgical debridement with extended margins was performed to get to sharp bleeding edges. The patient was changed from a wound vacuum to wet-to-dry dressing changes to allow easier inspection of the wound. He had daily bedside debridements in which any suspicious tissue was removed sharply and was sent for fungal culture. He was continued receiving intravenous liposomal amphotericin, and dissemination beyond the skin did not occur. A wound vacuum was replaced over the wound once the bedside debridements no longer showed hyphae, which was 7 days after the patient’s last formal operating room debridement (Fig. 3). The patient had a split-thickness skin graft at this time with full incorporation. He was in the hospital for a total of 16 days and received intravenous liposomal amphotericin for 14 days. The patient then took oral posaconazole 200 mg 4 times per day for 28 days.
At 1 year postoperatively, the patient had returned to all his activities but did have some difficulty with running. He was not found to have diabetes or to be immunocompromised during treatment.
Proceed to Discussion >>Reference: Whiteside W. Cutaneous mucormycosis after elective outpatient gastrocnemius recession for plantar fasciitis in an immunocompetent host: a case report. JBJS Case Connect. 2021 Sep 2;11(3).e21.003580.
Mucormycosis is the preferred term used to describe infections caused by the fungi belonging to the order Mucorales. Zygomycosis, an alternative term to describe these life-threatening infections, has become less accurate based on molecular studies in which taxonomic reclassification eliminated zygomycetes as a class. Rhizopus, Mucor, and Cunninghamella species account for up to 75% of mucormycosis cases.
Mucorales organisms are opportunistic and can be aggressive in the proper host spreading through angiolymphatics and vascular spaces leading to thrombosis, infarction, and tissue necrosis. In a review of 929 cases, the 3 major predisposing factors were diabetes (36%), malignancy (17%), and organ transplantation (7%). Primary cutaneous cases represent 7% to 16% depending on risk factors, but no underlying condition is seen in up to 50% of patients. According to the literature, there is a 4% to 15% mortality from a cutaneous localized infection and 26% to 29% in cutaneous with deep extension, and those with disseminated cutaneous mucormycosis have a mortality rate as high as 83% to 94%.
Cutaneous mucormycosis can develop in the immunocompetent host after substantial tissue trauma such as motor vehicle collisions or natural disasters in which the loss of normal skin integrity acts as an entry site for fungal infection. There have been cases in which several tornado survivors with penetrating trauma developed cutaneous mucormycosis. It has also been reported to occur after burns, spider bites, stings, and plant thorn injuries. More recently, it has been associated with COVID-19 in patients with diabetes because of the increase in dexamethasone use. Nosocomial infections accounted for 36% of infections in a review of 78 patients where cutaneous mucormycosis was associated with antifungal prophylaxis, wooden tongue depressors, ventilation systems, linens, bandages, medication patches, catheters, and ostomy bags. Once the cutaneous form becomes aggressive, it can threaten limbs by invading the subcutaneous tissue and can be threatening to adjacent fat, muscle, fascia, and bone.
Postoperatively in the orthopaedic patient, infections are typically Gram-positive organisms; therefore, high suspicion for cutaneous mucormycosis is necessary in the immunocompetent host. Necrotizing fasciitis and pyoderma gangrenosum are 2 other destructive conditions that should be in the differential diagnosis in atypical postoperative wound infections. There are no pathognomonic signs for a cutaneous mucormycosis infection, and it can mimic bacterial infection with erythema, vesicles, cellulitis, edema, fever, and pain. Clues that could be of use for the early identification of cutaneous disease is necrotic wound edges, if the wound continues to expand despite antibiotics, or if mold is observed on the wound edges. A deep surgical biopsy at the center of the lesion including subcutaneous fat and muscle and histopathologic examination and culture are essential for diagnosis. Communication with the hospital pathologist is paramount during this workup. It is essential to send for acid-fast bacilli and fungal cultures at the time of the surgical debridement to the microbiology laboratory. A direct potassium hydroxide examination can identify the fungus. The classic findings are nonseptated, broad, ribbon-like hyphae (Figs. 2-A and 2-B) with right-angle branching on histology. This is in contrast to Aspergillus, which shows acute angle branching and septate hyphae. Unfortunately, 72% of cultures do not yield a fungus. Therefore, DNA mapping with techniques such as next-generation sequencing or enzymatic template generation and amplification has begun to be used for a more rapid diagnosis of the exact microbe especially in culture-negative infections such as mucormycosis or periprosthetic joint infections.
Management includes aggressive surgical debridement to negative margins along with antifungal pharmacotherapy. Moran et al. reported that an average of 10 surgical debridements was necessary for eradication. Extensive surgical debridement is difficult to achieve because important structures are often adjacent to the necrotic tissue, especially with rhino-orbital and cerebral involvement. A surgical procedure may only yield partial remission, and, with cutaneous mucormycosis, amputation of the extremity can be the most definitive treatment. Correction of metabolic abnormalities, improvement in the immunosuppression status of the patient, and removal of devitalized tissues with antifungal therapy are the agreed-upon management of mucormycosis. A multidisciplinary team approach including nursing, surgeons, infectious disease, hospitalists, pathologists, pharmacy, laboratory staff, and social work is necessary.
Amphotericin B in its various formulations remains the drug of choice because of its broad-spectrum nature. Treatment regimens recommend 3 to 5 mg/kg of amphotericin B once per day for 6 to 8 weeks. The combination with oral posaconazole 200 mg 4 times per day can be used if renal toxicity occurs, if the patient is unresponsive to amphotericin B, or if discharge needs to be facilitated because the medication is oral. In a series of 91 patients with invasive disease, there was a 60% response rate at 12 weeks, and 21% achieved a stable disease state with this regimen. The duration of treatment is unknown, but case series have reported the administration of posaconazole from 2 to 34 months of treatment. Mucormycosis species are resistant to broad-spectrum azoles (except posaconazole) commonly used as empiric antifungal treatment, and discrimination between other fungi, Aspergillus and Candida, is necessary.
Cutaneous mucormycosis is caused by the damaged skin, which represents an entry site for fungal inoculation as occurs in trauma, but needs to be considered in any patient presenting with necrotic wound edges after a surgical procedure. When suspected, a full-thickness surgical skin biopsy and resection are necessary for the identification and treatment of this organism. An aggressive approach with immediate and often repeated surgical debridements and amphotericin B is essential for controlling the infection and preventing dissemination.
Reference: Whiteside W. Cutaneous mucormycosis after elective outpatient gastrocnemius recession for plantar fasciitis in an immunocompetent host: a case report. JBJS Case Connect. 2021 Sep 2;11(3).e21.003580.
What is the diagnosis?
Fungal hyphae consistent with mucormycosis
Necrotizing fasciitis
Pyoderma gangrenosum
Foreign material consistent with cotton fibers surrounded by necrotic tissue
Echinococcosis (hydatid cyst)

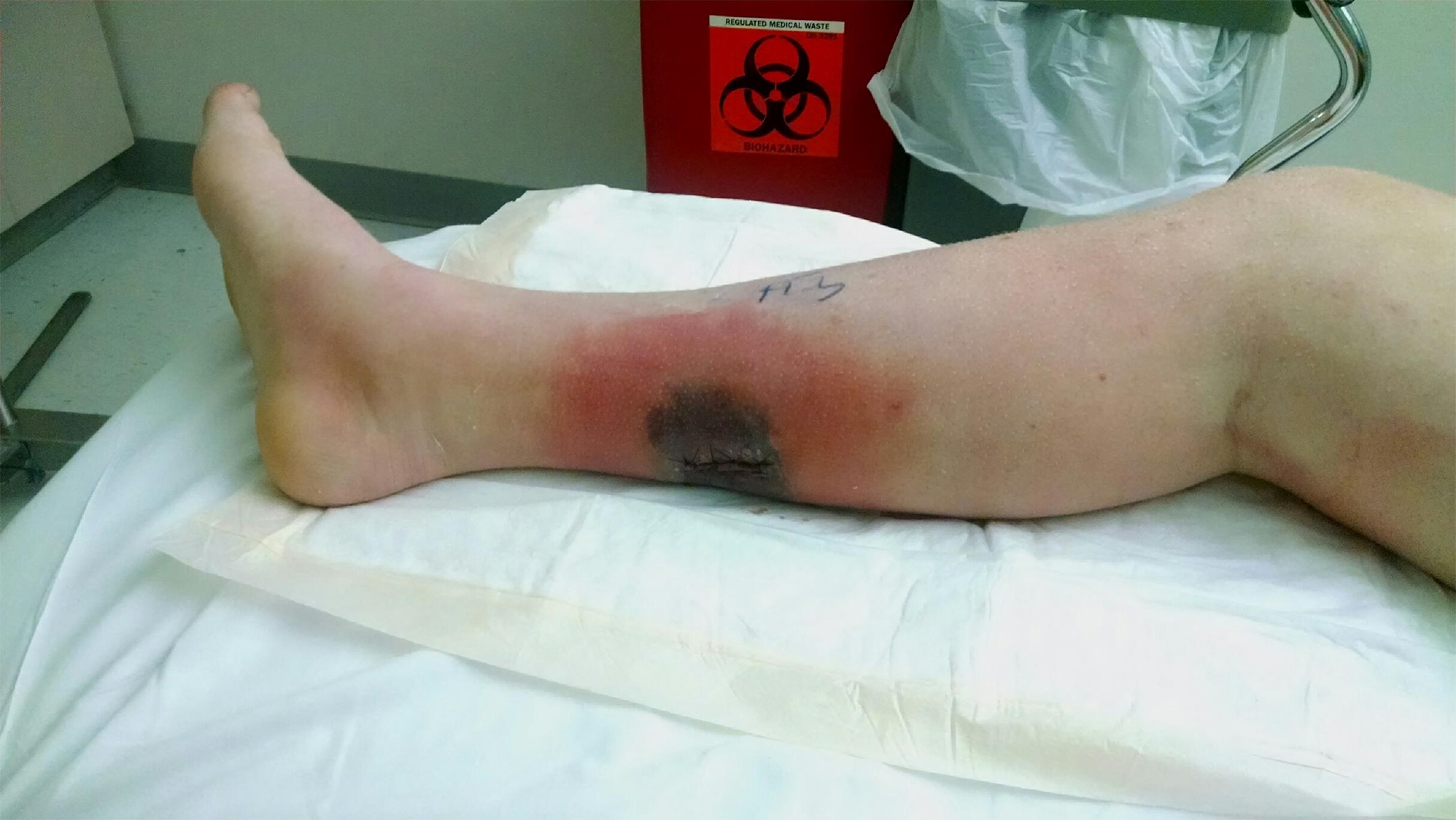
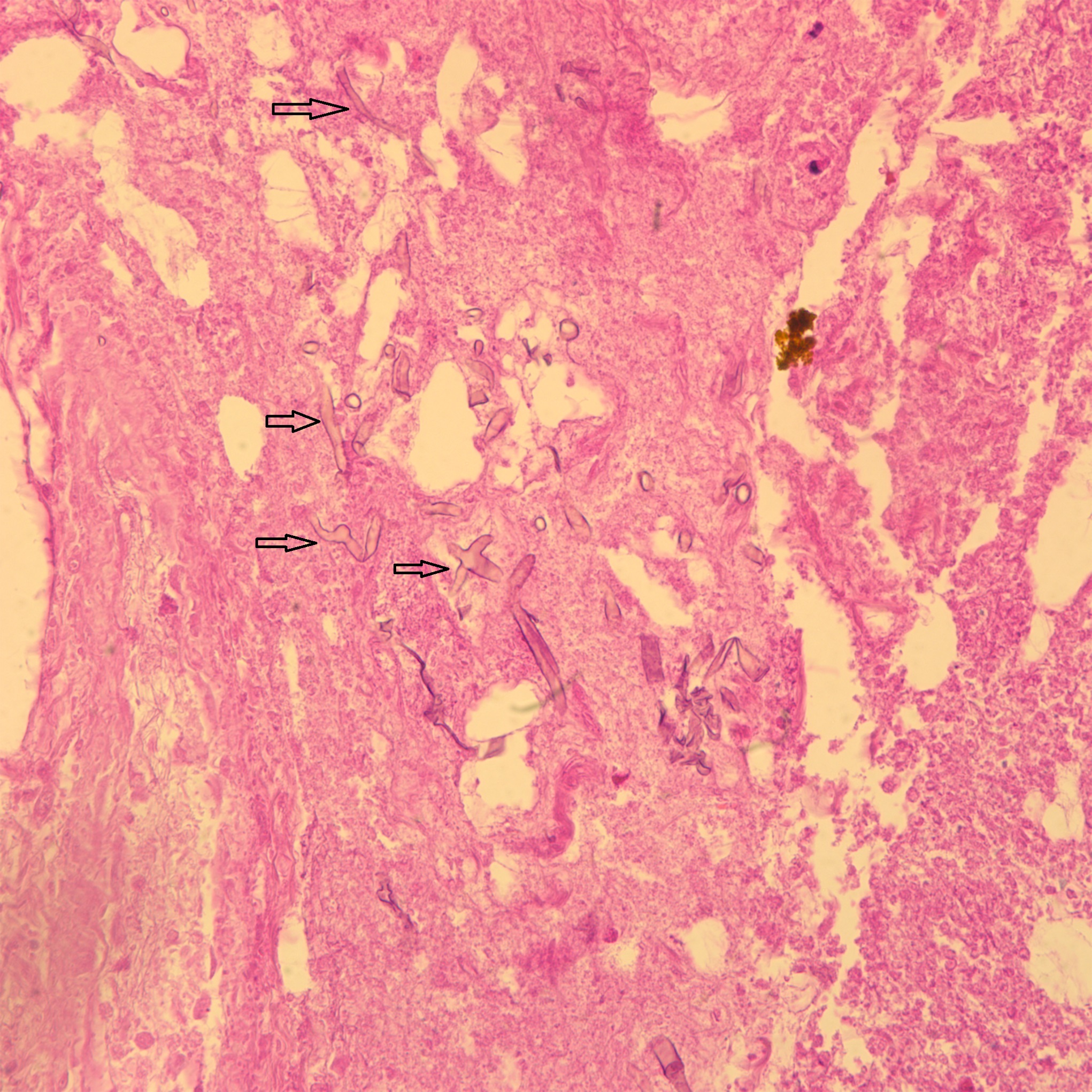
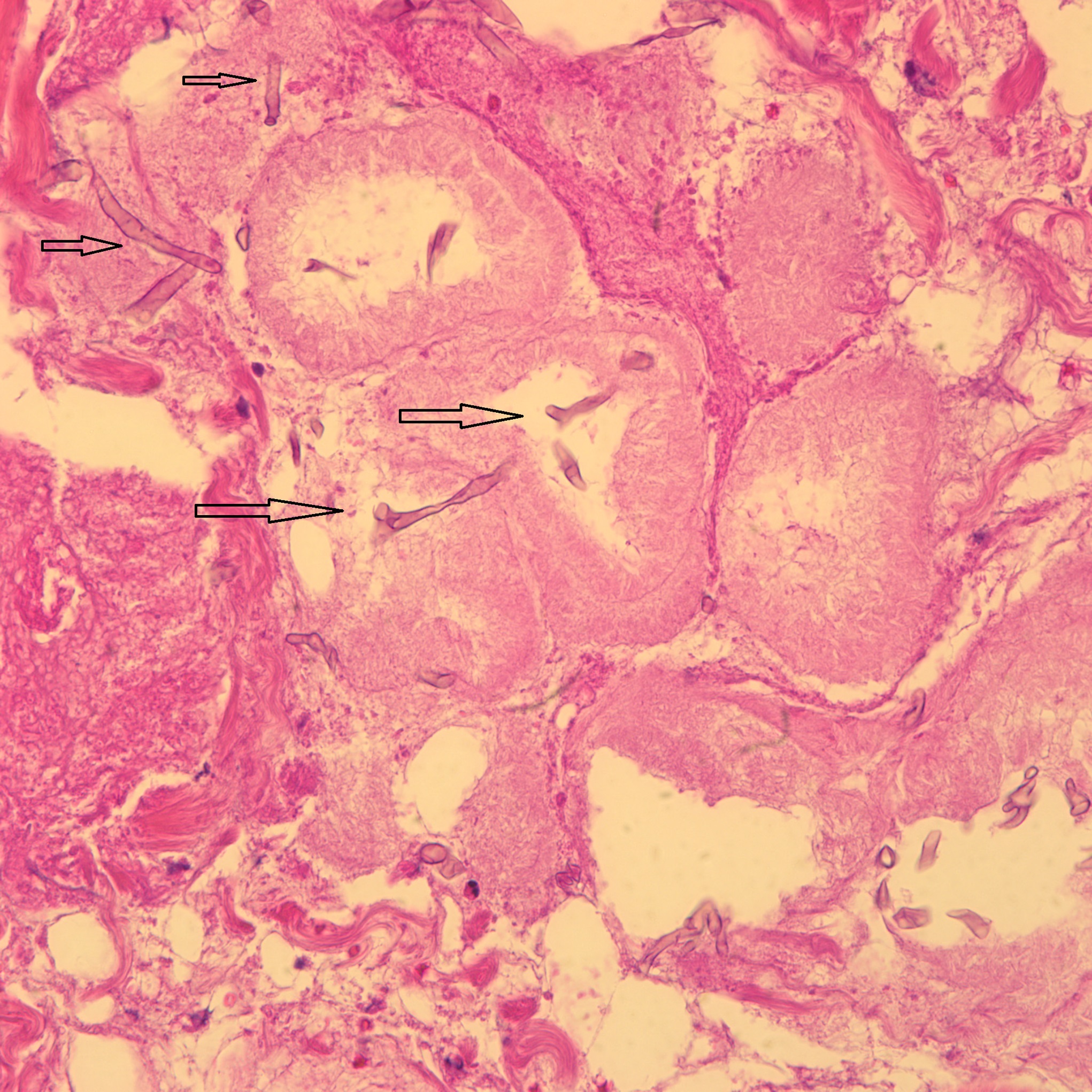
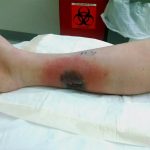 Fig. 1
Fig. 1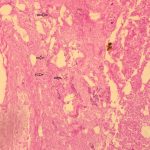 Fig. 2-A
Fig. 2-A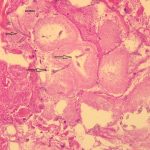 Fig. 2-B
Fig. 2-B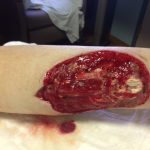 Fig. 3
Fig. 3