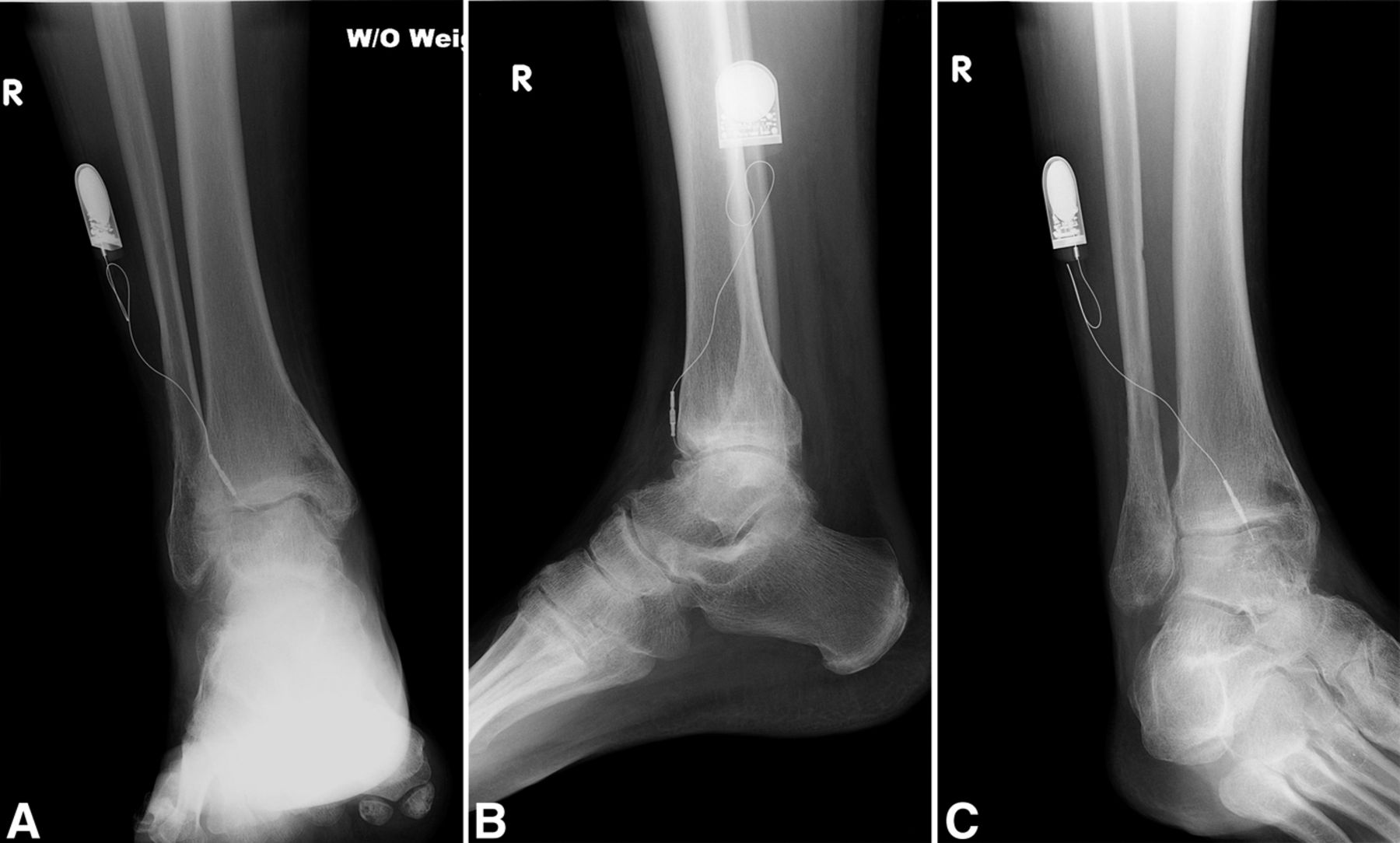
A sixty-year-old woman with no noteworthy medical history presented to our office with six months of left ankle pain. She had experienced a fall into a drained pool eleven months prior to the onset of pain. She had attempted a three-month trial of non-weight-bearing and physical therapy but had no improvement. On examination, the range of motion at the ankle and subtalar joints was preserved without any substantial pain. The initial radiographs demonstrated talar bone sclerosis without collapse and no adjacent joint arthritis (Figs. 1-A, 1-B, and 1-C). Magnetic resonance imaging (MRI) was obtained (Fig. 2). She subsequently wore a controlled ankle motion (CAM) walker boot with limited weight-bearing for five months.
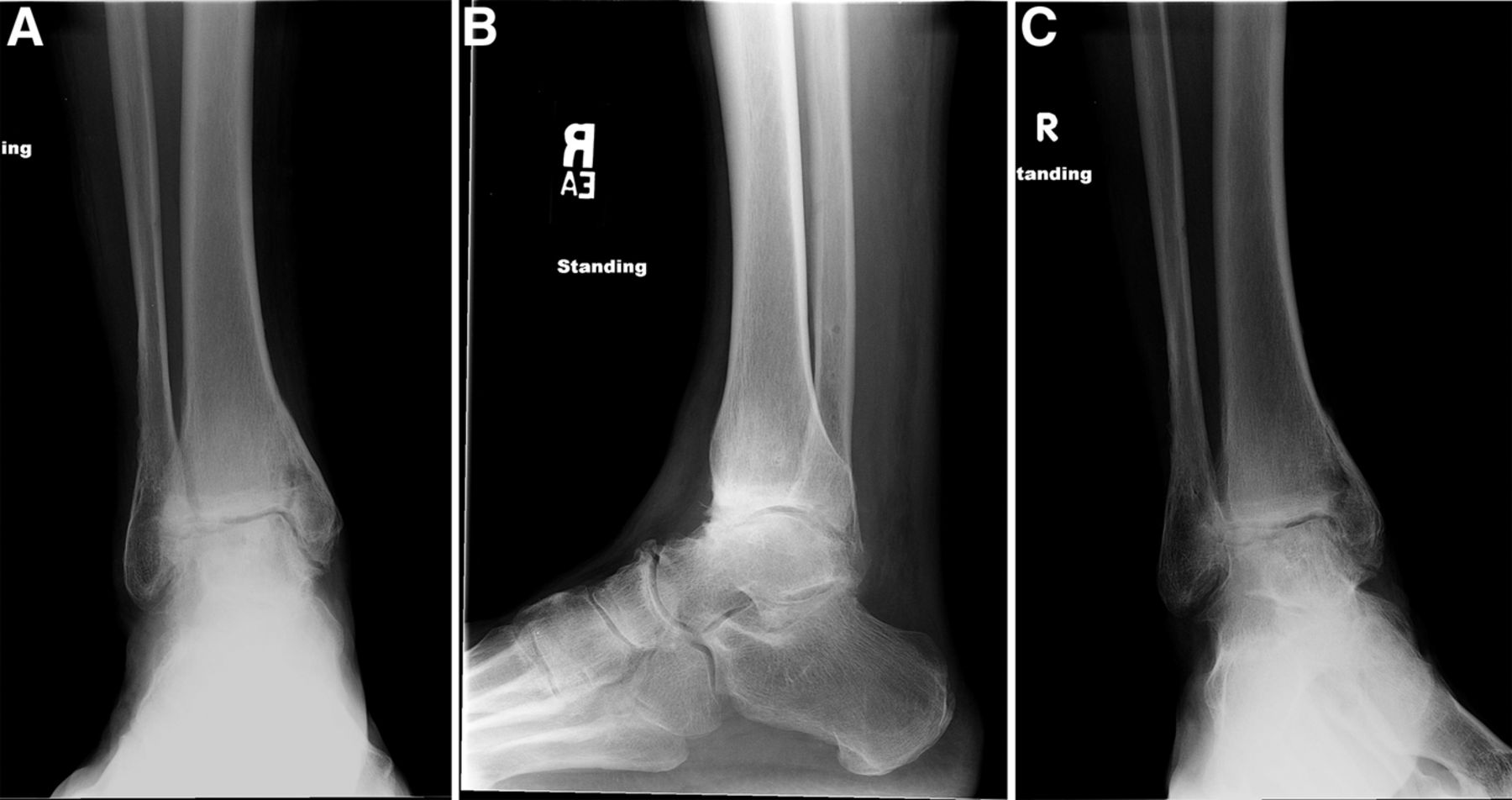
The MRIs showed osseous edema throughout the talus. The patient was unsatisfied with her progress and elected operative management to treat the osteonecrosis of the talus. Therefore, after eleven months of conservative therapy, the patient underwent ankle arthrotomy with drilling, placement of a tibial bone autograft, and placement of an internal bone stimulator (EBI OsteoGen; Biomet, Parsippany, New Jersey). An incision was made over the anterolateral aspect of the ankle since the battery would be placed on the lateral aspect of the ankle. The incision was taken down to the retinaculum to allow for visualization of the dorsum of the talus, the ankle joint, and the distal part of the tibia. An anterolateral 5-mm portal was then made in the neck of the talus to allow entry of the wire into the talus. Under fluoroscopic guidance, we were able to drill multiple holes in the necrotic portions of the talus. Next, we harvested bone from the distal part of the tibia. A burr hole was made into the distal part of the tibia with a medium-sized burr. Approximately, 5 cc of cancellous bone was harvested with use of a series of curets. The harvest site was then filled with calcium sulfate pellets. Next, we made a lateral incision about 6 cm proximal to the ankle joint in order to create a subcutaneous pouch for implantation of the battery. A subcutaneous pocket was tunneled to the ankle. The battery was implanted proximally, and the wire was taken subcutaneously to the level of the ankle. We placed the wire into three separate holes into the talus and packed it with the autograft bone from the distal part of the tibia. We then applied bone wax to the surface, irrigated, obtained final radiographs, and closed the incision in layers. The patient was made non-weight-bearing for a total of six weeks. Initial postoperative radiographs were obtained at two weeks (Figs. 3-A, 3-B, and 3-C). At six weeks, the patient began wearing a CAM boot and was allowed to bear weight as tolerated. She did well postoperatively without any complications. There was good incorporation of the autogenous bone graft to the body portion of the talus (Figs. 4-A, 4-B, and 4-C). The patient underwent battery and wire removal without complication six months after the initial surgery. At one year postoperatively, she was doing well and had returned to full duty at work (Figs. 5-A, 5-B, and 5-C). She was able to walk without any assistive device or orthosis. She was satisfied with the outcome.
Proceed to Discussion >>Reference: Holmes GB Jr, Wydra F, Hellman M, Gross CE. A unique treatment for talar osteonecrosis: placement of an internal bone stimulator: A case report. JBJS Case Connect. 2015 Jan 14; 5(1):e4.
There are many treatment options for talar osteonecrosis in the orthopaedic surgeon’s armamentarium; however, the results are often unpredictable. There is a wide spectrum of conservative treatments, ranging from non-weight-bearing and modified weight-bearing to wearing a patellar tendon-bearing brace and extracorporeal shockwave therapy (ESWT). Most surgeons advocate at least a trial of conservative measures prior to operative intervention. In a review of the literature, the only randomized control trial regarding conservative therapy was a prospective study by Zhai et al. that evaluated the use of ESWT to treat talar osteonecrosis. They illustrated excellent outcomes with respect to American Orthopaedic Foot & Ankle Society(AOFAS) scores, visual analogue scale scores, and MRI edema patterns eighteen months after ESWT had been performed once a week for three to five cycles. Other surgeons have used vascularized bone grafts to attempt to revascularize the talus. These procedures have shown promising results, with good/excellent outcomes in 90% and 83% of patients with nonvascularized and vascularized bone grafts, respectively, and excellent outcomes with regards to pain and function. However, the data are limited to a small number of patients. Core decompression has also provided a viable alternative to arthrodesis. The core decompression studies exhibited improvements in validated outcome measures, with increases in Mazur and AOFAS scores. These improvements were seen in both early and late stages of osteonecrosis. Bone stimulators work to alter the electrochemical properties of traumatized bone. Traumatized bone has large areas of cellular activity and metabolism that generate a negative bioelectric potential across cellular membranes. Bone has large amounts of calcium phosphate crystals that, when stressed, tend to generate piezoelectric voltage (tension corresponds to electropositivity, and compression corresponds to electronegativity). Therefore, applying an exogenous electric current across stressed bone (e.g., osteonecrotic bone) may modify osseous healing and remodeling. In fact, in vivo and in vitro studies have shown that electrical stimulation can cause osteoprogenitor cells to differentiate into osteoblasts. To our knowledge, there is currently no research on the use of adjuvant internal bone stimulators in the treatment of osteonecrosis of the talus. The first human use of direct current bone stimulators was in 1972 with tibial pseudoarthroses. Direct current implantable electrostimulation has been used successfully in the foot and ankle for at-risk hindfoot fusions, Charcot ankle arthrodesis, fracture nonunion, revision ankle arthrodesis, and various at-risk forefoot and hindfoot arthrodeses. Most of these studies define “at-risk” patients as those who are immunocompromised, those who smoke, or patients with diabetes, neuropathy, or vasculopathy. These Level-IV studies suggest that direct current devices may be an adjunct in fusion or nonunion healing. There are many theoretical advantages of using an implantable direct current bone stimulation device. It ensures patient compliance, and its constant stimulation is maximally directed at the surface of interest. On the other hand, a secondary procedure might be necessary for removal of the battery because of soft-tissue irritation, infection, or pain. This case report does have its limitations in suggesting a treatment algorithm. Because our patient underwent drilling, bone-grafting, and implantation of a stimulator, it is not clear which was the essential component of treatment. A follow-up period of fourteen months is relatively short. Future studies should include a randomized control trial or at least a cohort study with and without electrostimulation therapy in people with unsuccessful conservative therapy. This case report serves to showcase the combination of three treatments with existing technology that may be useful to treat talar osteonecrosis. To the best of our knowledge, no previous case report has discussed the treatment of osteonecrosis with an implantable bone stimulator. The bone stimulator is easy to implant during other open treatments for talar osteonecrosis and may prove to be a useful adjunct with current treatment strategies.
Reference: Holmes GB Jr, Wydra F, Hellman M, Gross CE. A unique treatment for talar osteonecrosis: placement of an internal bone stimulator: A case report. JBJS Case Connect. 2015 Jan 14; 5(1):e4.
Osteonecrosis of the talus
Osteochondritis dissecans of the talus
Osteoid osteoma of the talus
Osteoblastoma of the talus
Lymphoma of bone involving the talus

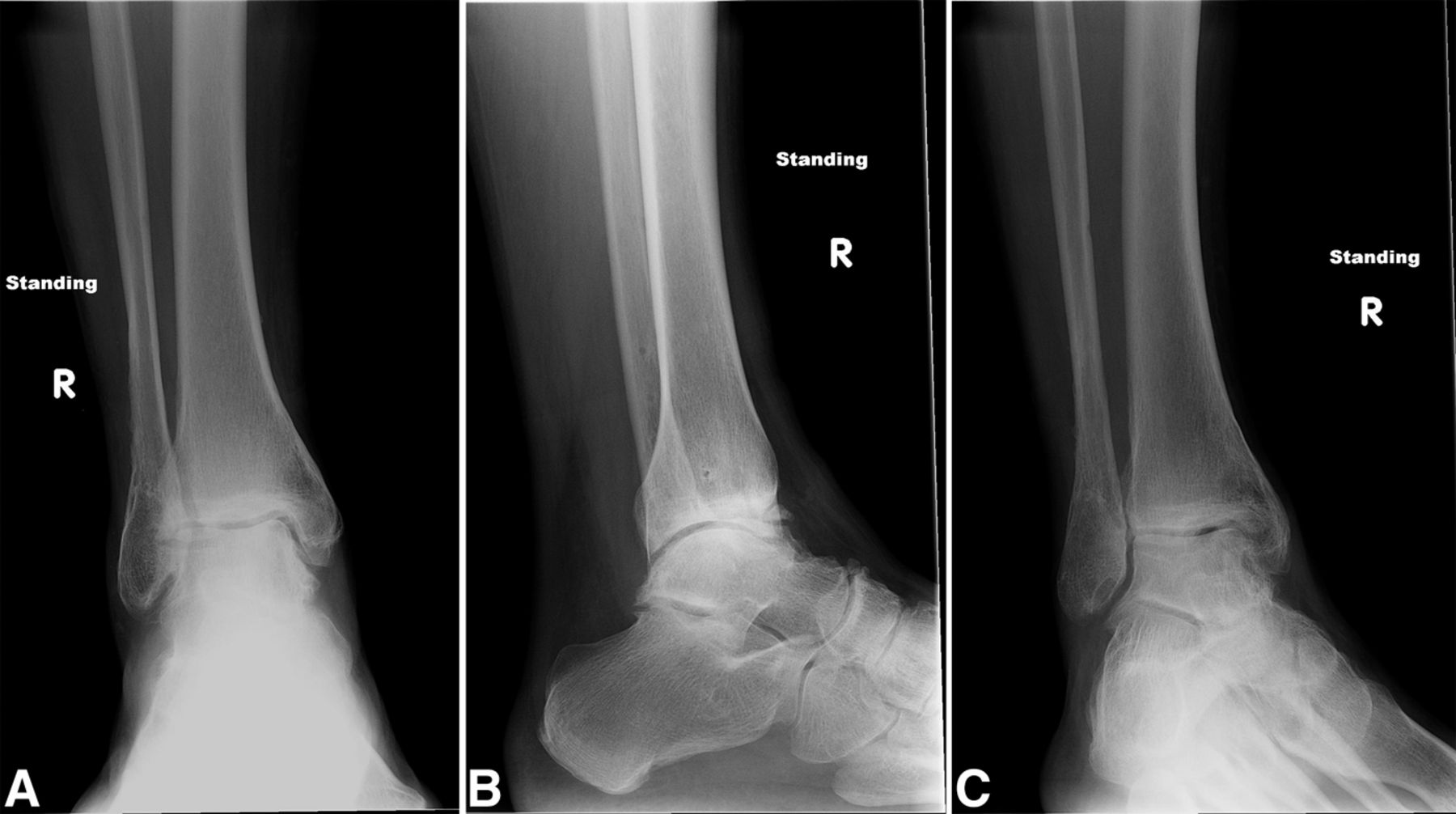
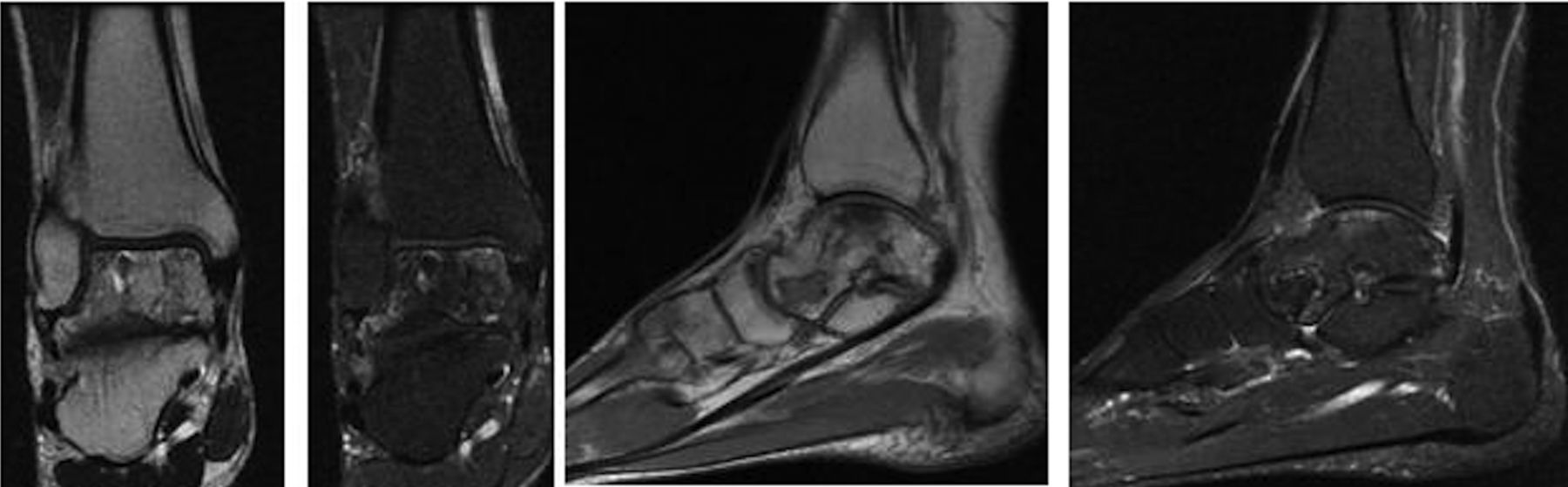
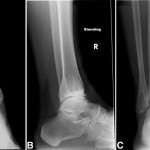 Fig. 1
Fig. 1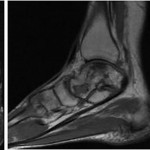 Fig. 2
Fig. 2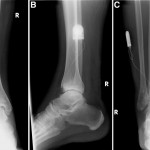 Fig. 3
Fig. 3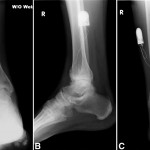 Fig. 4
Fig. 4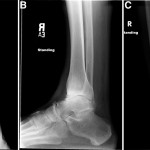 Fig. 5
Fig. 5