A Thirty-five-Year-Old Female with Iliac Vein Occlusion and Spinal Stenosis
July 6, 2011
A thirty-five-year-old woman presented to the emergency department with the acute onset of bilateral lower extremity edema and severe lower extremity pain. The patient had a one-year history of bilateral lower extremity pain and activity-related weakness consistent with neurogenic claudication. The medical history was notable for hypothyroidism as well as for a Maverick metal-on-metal total disc replacement at the L4-L5 disc level, which had been performed three years previously. She had no known allergies to metals that would have indicated a possible hypersensitivity reaction. Physical examination revealed massive bilateral lower extremity swelling that was worse on the left side as well as pain throughout the legs, consistent with the diagnosis of deep venous thrombosis. The lower extremities were diffusely weak and had, at best, grade-4 of 5 strength in all lower muscle groups. The lower extremity reflexes were diminished, and there was no clonus. The patient was admitted to the vascular surgery service. Vascular duplex ultrasound revealed extensive deep venous thrombosis throughout the left lower extremity, the iliac venous system, and the inferior vena cava. A computed tomography scan showed a large mass occluding the left iliac vein and the inferior vena cava as well as extending posteriorly behind the L4-L5 interspace, into the epidural space (Figs. 1 and 2). The mass measured 15 mm from anterior to posterior in front of the L4-L5 space and 50 mm from cephalad to caudad at its largest point just left of the midline in front of L4 and L5. The mass appeared to originate at the L4-L5 level. The erythrocyte sedimentation rate and the C-reactive protein level were elevated to 32 mm/hr (normal, 0 to 20 mm/hr) and 10.2 mg/L (normal, 0 to 0.8 mg/L), respectively. The white blood-cell count was normal at 8.3 × 109/L (normal, 4.0 to 12.0 × 109/L). A bone scan revealed mild tracer uptake anterior to the sacroiliac joints in all three phases but did not indicate any uptake in the region of the disc replacement. A myelogram showed spinal stenosis at the level of the L4-L5 disc space, with a near complete block of the flow of contrast medium. The prosthesis appeared to be in a satisfactory position, with no signs of osteolysis or loosening. Initial treatment was directed at limiting enlargement and further extension of the venous clots. The patient was placed in compression stockings, both legs were elevated, and anticoagulation with a heparin drip was started. Because of the size of the clots, thrombolysis with a hemolytic agent was not deemed a suitable treatment modality. The orthopaedic spine service was consulted for further evaluation of the relationship of the mass to the total disc replacement. After the insertion of a vena cava filter, the patient underwent a posterior spinal decompression, a tissue biopsy was performed (Fig. 3), and specimens were obtained for culture. Grossly, the mass had a reddish-gray color, with no obvious pus being present.
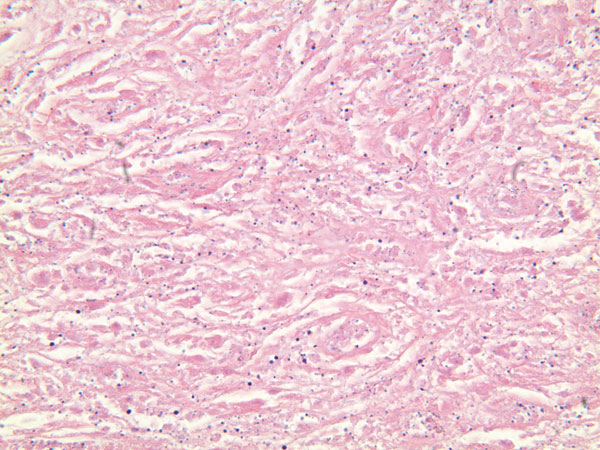
A Gram stain and frozen-section analysis of the abnormal tissue revealed no evidence of either infection or malignancy. The mass was consistent with a host reaction to a foreign body. After debulking of the mass at L4 and L5, a posterior spinal decompression and arthrodesis was performed. The mass extended from the L4 nerve root sleeve to the top of the L5 nerve root and was adherent to the dura at the level of the L4-L5 disc space while wrapping nearly circumferentially around the dural sac to the dorsal midline of the spine. Following excision of the mass, the dura required extensive reconstruction. The final pathology report described the tissue as benign and reactive and consistent with a large granuloma. There were uniformly sized, shaped, and colored particles within the granulation tissue, indicative of wear debris (Fig. 3). All wound and tissue cultures were negative for infection. Eighteen months after surgery, the mass had not enlarged as determined with serial ultrasound examinations. The patient had a steady return of bilateral lower extremity strength and could walk independently. The patient had minimal requirements for narcotic pain medication, but she had aching discomfort and swelling of the lower extremities, consistent with a post-phlebotic syndrome. The vena cava filter remained in place, and, at the time of the latest follow-up, the patient was receiving chronic anticoagulation with warfarin therapy.
Proceed to Discussion >>Reference: Berry MR, Peterson BG, Alander DH. A granulomatous mass surrounding a maverick total disc replacement causing iliac vein occlusion and spinal stenosis. A case report. J Bone Joint Surg Am. 2010;92:1242-5.
There have been numerous reports of granulomatous masses surrounding total joint implants. To date, we are aware of no reported cases of a granulomatous mass as a host response to a metal-on-metal total disc arthroplasty. Motion wear across a prosthesis bearing surface is associated with the formation of particulate wear debris, loss of joint height, and ultimately failure. More importantly, the particulate debris can induce an inflammatory response mediated by various cytokines, including tumor necrosis factor-alpha 1 (TNF-alpha1), matrix metalloproteinase-1 (MMP1), interleukin-1 (IL-1), interleukin-6 (IL-6), and prostaglandin E2 (PGE2). This inflammatory response may lead to pain, osteolysis, pannus formation, and loosening of the implant. Corrosion products (metal ions and/or their precipitates) can be generated at any metal surface but occur most commonly at the metal-on-metal interfaces of modular implants. In a study by Zeh et al., serum metal ion concentrations in patients with one and two-level Maverick disc prostheses met or exceeded those in patients with total hip replacements with metal-on-metal bearing surfaces. However, the known granulomatous response to metallic wear debris in prosthetic joints and a pathologic diagnosis consistent with a granuloma and metal wear particles led us to conclude that this granuloma was the result of metallic wear debris generated by the Maverick disc. The case of our patient raises some important clinical questions. Because the implants are so new, there is a dearth of information regarding the timing, length, and best method of clinical follow-up. Routine radiographs may not detect a subtle granulomatous mass surrounding an implant. Both computed tomography and magnetic resonance images are limited by metal-induced artifact and are not practical for routine screening. The combination of the slow onset of symptoms, the metal artifact distortion during radiographic imaging, and the extensive venous compression proved to be clinically challenging. After undergoing decompression of the granuloma, our patient has had regular clinical evaluations for recurrent venous thrombosis and neurologic decline. Duplex ultrasound, in addition to radiographs, has been used to monitor this patient. The ultrasound, consisting of both B-mode ultrasound and color Doppler ultrasound, has provided ongoing measurement of the granuloma size, venous obstruction, and flow abnormalities. On the basis of the case of our patient, we believe that long-term clinical follow-up evaluations should be done for all patients with this device. Particular attention should be paid to subtle progressive pain and signs of vascular or neurologic compromise as possible early warnings of a developing granuloma. Additional investigation needs to be done regarding appropriate strategies to avoid and, if necessary, to treat this challenging complication of a metal-on-metal total disc replacement.
Reference: Berry MR, Peterson BG, Alander DH. A granulomatous mass surrounding a maverick total disc replacement causing iliac vein occlusion and spinal stenosis. A case report. J Bone Joint Surg Am. 2010;92:1242-5.
Late low-grade bacterial infection involving the total disc implants
Local reaction to wear debris at total disc implant
Unrecognized displacement of part of one component of total disc implant
Late fungal infection with granuloma formation at total disc implant level

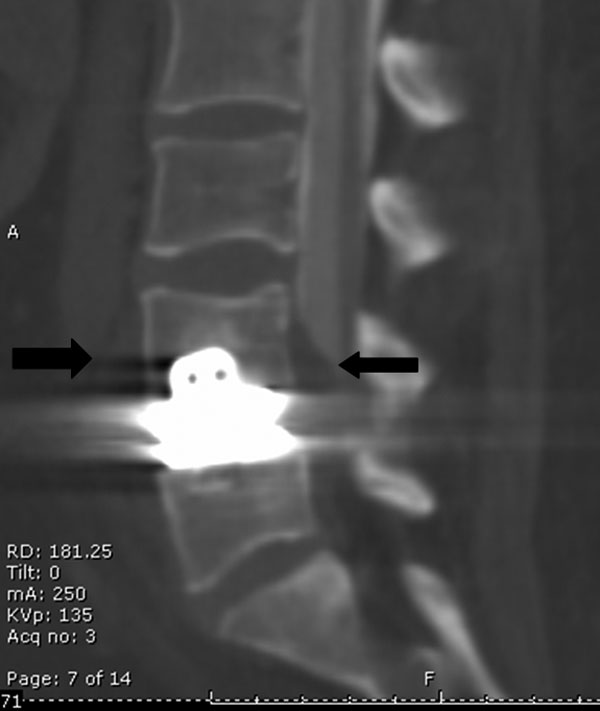


 Fig. 1
Fig. 1 Fig. 2
Fig. 2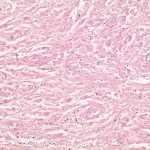 Fig. 3
Fig. 3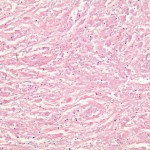 Fig. 3
Fig. 3