A Thirty-Year-Old Man with Three-Year Progressive Hip Pain
February 4, 2015
A thirty-year-old man with a past medical history noteworthy for Mediterranean thalassemia minor presented with a three-year history of progressive severely debilitating left hip pain and stiffness. He noted a progressive increase in hand size and limited range of motion in the left forearm. A metabolic bone specialist had performed extensive laboratory and imaging tests without revealing a diagnosis and had referred him to our practice because of an ankylosed hip. The initial physical examination was notable for a posture compromised by fixed hip flexion and a markedly antalgic gait. The left hip demonstrated a flexion contracture of approximately 40° and range of motion of 10° of internal rotation, 20° of external rotation, and 20° of abduction, all characterized by clinical impingement with firm painful end points. He demonstrated osseous excrescences in the hands and lacked supination and pronation of the left forearm. The patient had undergone extensive imaging at different clinical sites. Radiographs demonstrated extensive periarticular heterotopic bone formation and dense sclerosis of the femoral head and acetabulum of both hips, with near ankylosis of the left hip (Fig. 1-A). Computed tomography (CT) of the left hip confirmed generalized osteosclerosis and revealed patchy areas of trabecular rarefaction, osseous excrescences, and extensive hip osteophytosis (Fig. 1-B). The lumbar spine was similarly characterized by diffuse osteosclerosis (Fig. 2). Radiographs of both elbows revealed similar bone formation with complete synostosis of the left elbow (Figs. 3-A and 3-B). Radiographs of both hands also demonstrated substantial periostitis deformans (cloaking undulating periostitis) (Figs. 4-A and 4-B). A dual x-ray absorptiometry (DXA) scan and an extensive metabolic workup were obtained to evaluate the etiology of the diffuse osteosclerosis. The DXA scan of the lumbar spine, hip, and distal parts of the radii revealed Z-scores of +6.2, +3, and –1.2, respectively. A whole-body bone scintigram revealed diffusely increased uptake (Fig. 5). Laboratory data were remarkable for 25-hydroxyvitamin D deficiency (17 ng/mL; normal, 30 to 100 ng/mL), elevated bone alkaline phosphatase (70 μg/L; normal, <20 μg/L), and increased urinary N-terminal telopeptide (129 nmol/mmol; normal, 21 to 66 nmol/mmol). Laboratory tests measuring serum calcium, phosphorus, creatinine, parathyroid hormone, thyroid hormone, and twenty-four-hour urine creatinine were all within normal limits.
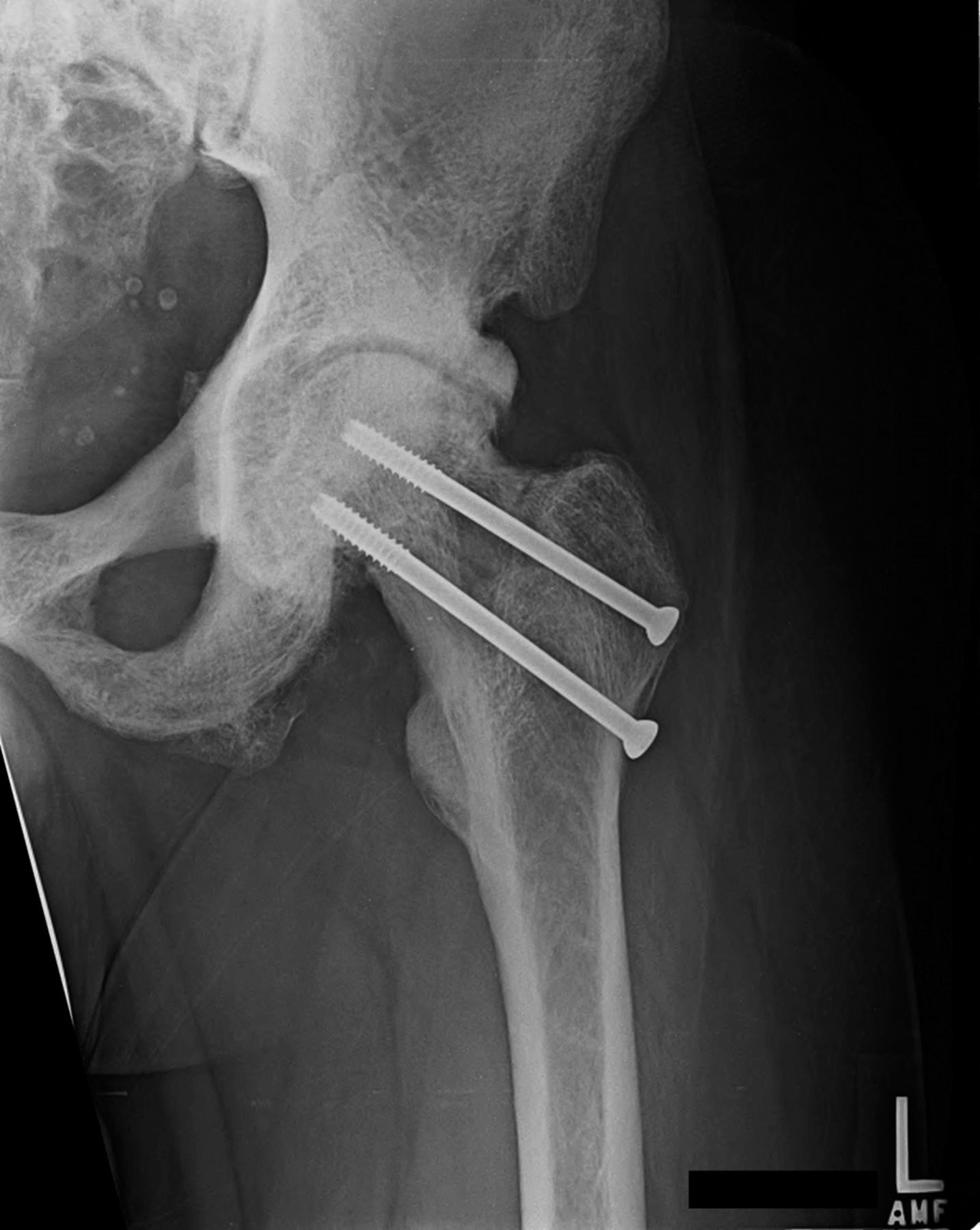
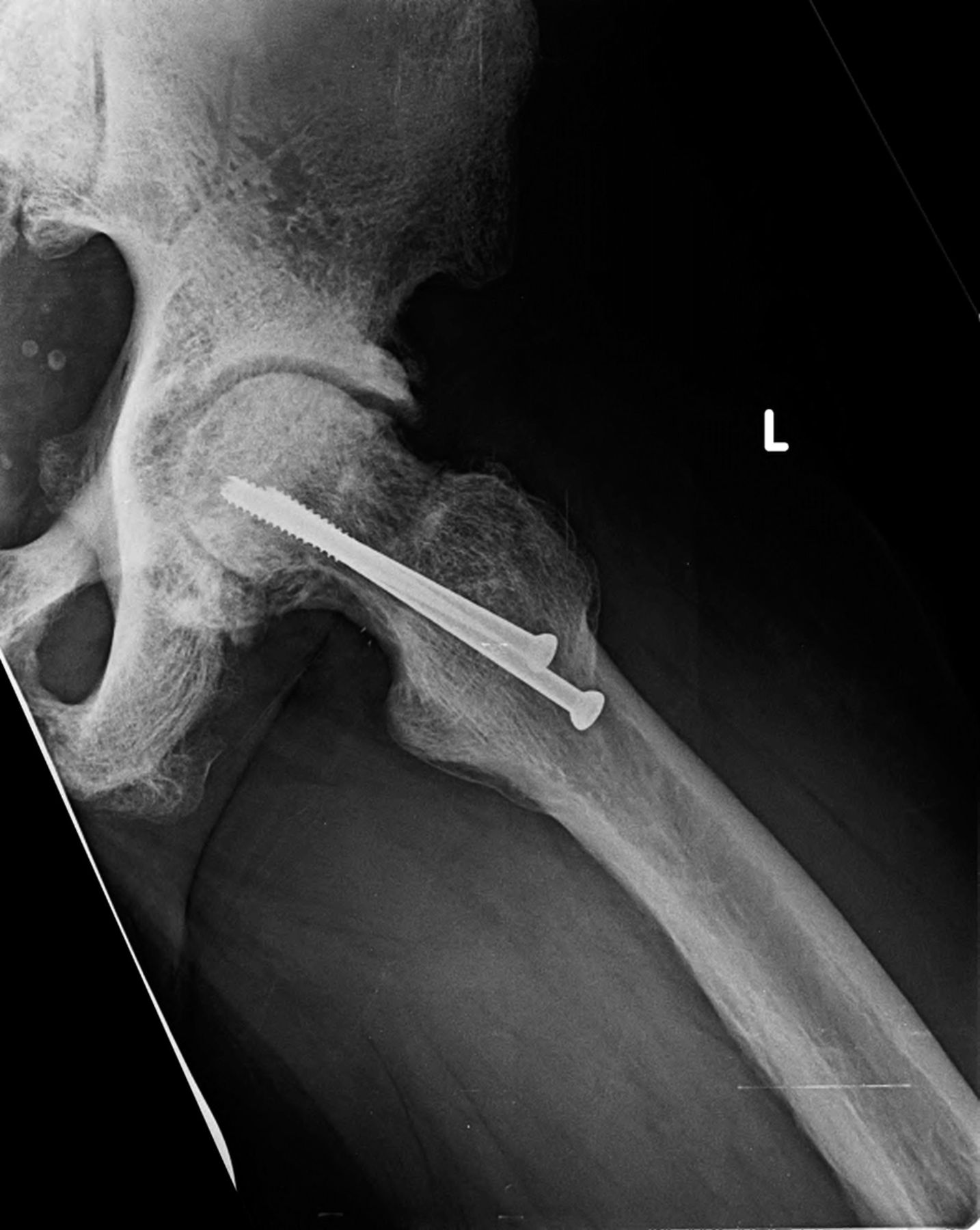
Conservative treatment with physical therapy and anti-inflammatory medication provided minimal relief; therefore, the patient underwent joint-preserving surgery to address the left hip ankylosis. Through a direct anterior approach, the hip capsule was released and extensive osteochondroplasty of the femoral head and neck was performed. Given the previously noted areas of femoral neck rarefaction and the potentially decreased ability for sclerotic bone remodeling, two prophylactic 6.5-mm cannulated screws were placed in the femoral neck. At the conclusion of the case, the hip demonstrated 0° to 120° of flexion, 30° of internal rotation, 70° of external rotation, and 45° of abduction. The patient was allowed to bear weight as tolerated. At one month postoperatively, the course was complicated by a low-energy nondisplaced femoral neck fracture around the screws, which was managed with limited weight-bearing (Fig. 6-A). Follow-up radiographs at six months postoperatively demonstrated healing and suggested decreased sclerosis (Figs. 6-B and 6-C). At the six-month follow-up, the patient was walking without assistive devices with a smooth, minimally antalgic gait pattern and an upright posture. The left hip range of motion was improved compared with the preoperative baseline, with 10° to 120° of hip flexion, 10° of internal rotation, 80° of external rotation, and 10° of abduction. An additional postoperative workup was performed to identify the cause of the extensive heterotopic bone and diffuse sclerosis. The initial workup had focused on the differential for diffuse osteosclerosis, which includes hemoglobinopathies, renal osteodystophy, Paget disease, pyknodysostosis, osteopetrosis, dysplasias, hypothyroidism, mastocytosis, myelofibrosis, and skeletal metastases. Given the rarity of skeletal fluorosis in the United States, the patient’s repeated denial of substance abuse, and the rapidity of onset, fluorosis was not considered in the original differential diagnosis. After ongoing discussion with the patient, he ultimately disclosed substantial recreational inhalation of Dust-Off (Falcon Safety Products, Branchburg, New Jersey), which contains 1,1-difluoroethane (DFE), over the preceding three years. Confirmatory serum fluoride levels were substantially elevated at 18.8 mg/L (normal, 0.20 to 3.21 mg/L). Attempts to obtain a gold-standard bone biopsy were aborted after we tried to penetrate the iliac crest with a Bordier trephine (5 mm) several times but were unsuccessful. The patient was ultimately lost to follow-up one year after surgery.
Proceed to Discussion >>Reference: Cohen E, Hsu RY, Evangelista P, Aaron R, Rubin LE. Rapid-onset diffuse skeletal fluorosis from inhalant abuse: a case report. JBJS Case Connect. 2014 Nov 26;4(4):e108.
Although skeletal fluorosis is uncommon in the United States, it is important to include it in the differential diagnosis for osteosclerosis and joint pain. Skeletal fluorosis may also present with dental mottling, stiffness, fracture, or neurologic complications. The slow development of skeletal fluorosis over decades from chronic exposure is well described. Cases with rapid onset have previously only been associated with fungal prophylaxis with voriconazole. There recently has been one reported case linked to abuse of another inhalant, dichlorodifluoromethane (Freon). Dicholorodifluoromethane is a chlorofluorocarbon (CFC) that historically has been used as a refrigerant and aerosol propellant that depletes the ozone layer, whereas DFE is an ozone-friendly fluorocarbon that has largely replaced CFCs. Our patient presented with hip pain and stiffness; the primary symptom of the patient who abused dichlorodifluoromethane was painless masses. To the best of our knowledge, ours is the first report to date describing the development of rapid-onset diffuse skeletal fluorosis from DFE inhalation. Inhaled DFE accumulates in high levels in the brain, causing euphoria, intoxication, and confusion. Reported complications of inhalant abuse include angioedema, seizures, cardiac arrhythmia, unconsciousness, and sudden death. Hahn et al. performed a postmortem analysis of a driver who was fatally injured in a motor vehicle accident after DFE inhalation. Laboratory tests revealed elevated DFE levels of 85.6 mg/L in the aortic blood, 40.3 mg/L in the pulmonary arterial blood, and 71.0 mg/L in the urine. It has been estimated that ingestion of 10 mg of fluoride per year for ten years is necessary to develop preclinical skeletal fluorosis. Our patient presented with limited hip joint movement, consistent with phase-III crippling skeletal fluorosis (>8400 mgF/kg ash concentration). Currently, there is no data that evaluates direct inhalation of fluoride agents and bone uptake. Our patient, however, presented with the combination of DFE inhalation, elevated blood fluoride levels, and skeletal fluorosis. With the increasing use of DXA scans in the United States, the first quantitative evidence of skeletal fluorosis or other osteosclerotic disorders may be an increase in bone mineral density. Our patient’s DXA scan demonstrated elevated Z-scores similar to previously reported cases of skeletal fluorosis. Chronic exposure to fluoride increases osteoblastic activity, bone formation, and bone alkaline phosphatase. Osteoblastic activity may be enhanced because of fluoride’s effects on local growth factors or fluoride’s inhibition of osteoblastic acid phosphatase, which in turn increases cellular tyrosyl phosphorylation, responsible for stimulating bone growth. Fluoride has been pursued as a potential treatment for osteoporosis for over thirty years. A meta-analysis of fluoride in postmenopausal women showed that although fluoride increases the bone mineral density of the lumbar spine, it does not decrease the risk of vertebral fractures; increased doses actually increase the risk of nonvertebral fractures. Therefore, DXA scans in the setting of skeletal fluorosis must be interpreted with caution as there may be an increased risk of fracture from disordered osseous remodeling. Our patient presented with the most recognizable radiographic findings of skeletal fluorosis: osteosclerosis, cloaking periostitis, osteophytosis, and ligamentous calcification. Wang et al. reviewed the radiographic features of 127 patients with endemic fluorosis and found osteosclerosis in 43%, diaphyseal widening in 28%, and calcification of the ligamentous attachments in 89%. Other radiographic findings were growth lines (70%) and osteopenia (40%). Mithal et al. found that patients with osteopenia had substantially lower dietary calcium intake compared with patients with normal or osteosclerotic radiographs, suggesting that calcium intake plays a role in the radiographic presentation of endemic fluorosis. If the diagnosis of skeletal fluorosis is suspected, serum fluoride levels and twenty-four-hour urine fluoride and creatinine levels will support the diagnosis, but the gold standard is quantitative bone ash fluoride analysis from a bone biopsy. The medical treatment of skeletal fluorosis starts with removal of the offending agent, followed by repletion of vitamin D and possibly calcium. Kurland et al. reported hypercalciuria and kidney stones during skeletal unloading of fluoride; therefore, the monitoring physician should be cautious with calcium supplementation and ensure adequate hydration. Following medical stabilization, resection of impinging osteophytes and synostotic lesions to restore motion, as well as prophylactic fixation of impending fractures, should be considered as part of the overall management strategy.
Reference: Cohen E, Hsu RY, Evangelista P, Aaron R, Rubin LE. Rapid-onset diffuse skeletal fluorosis from inhalant abuse: a case report. JBJS Case Connect. 2014 Nov 26;4(4):e108.
Pycnodysostosis
Melorheostosis
Skeletal fluorosis associated with inhalant abuse
Osteopetrosis
Renal osteodystrophy

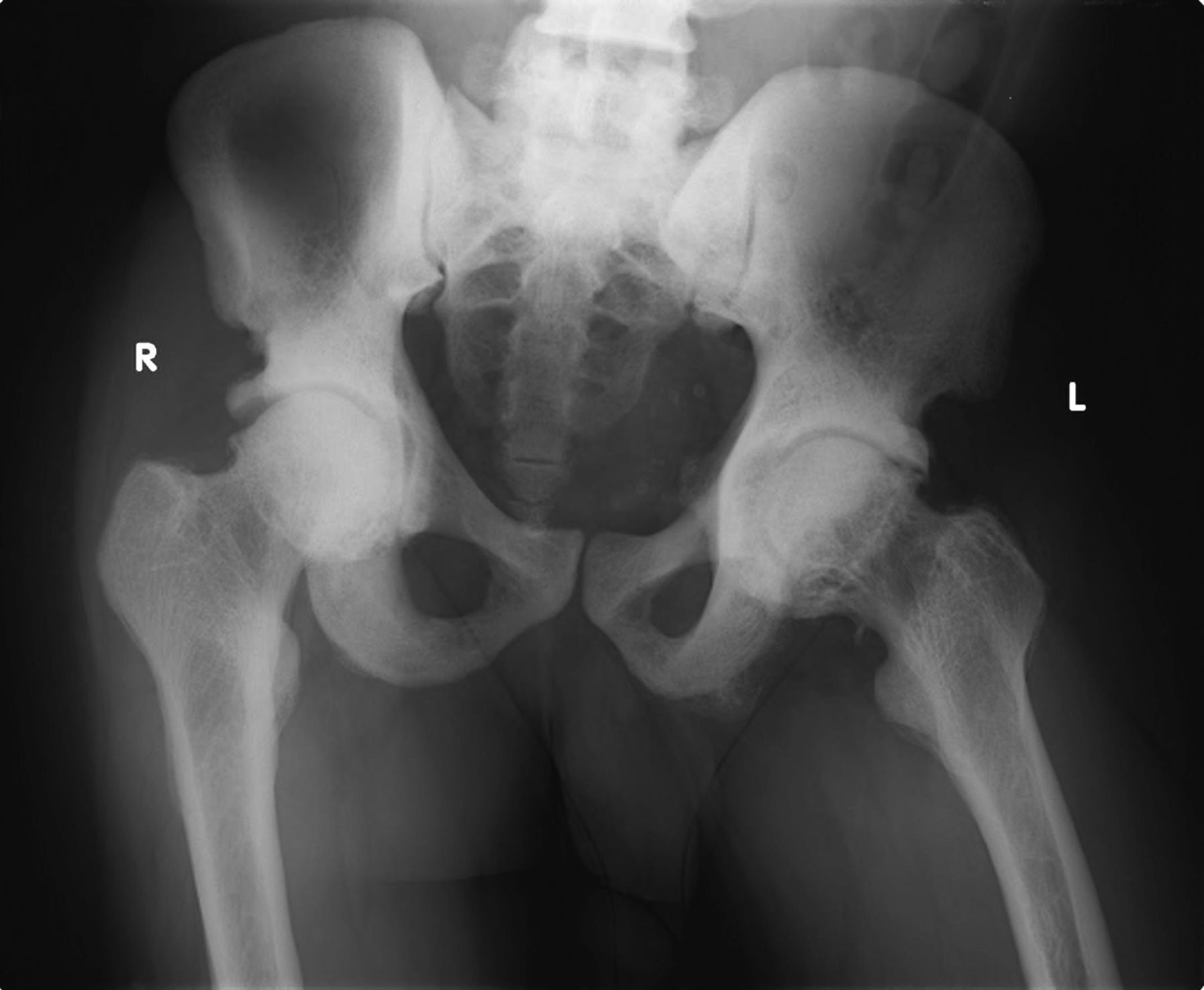
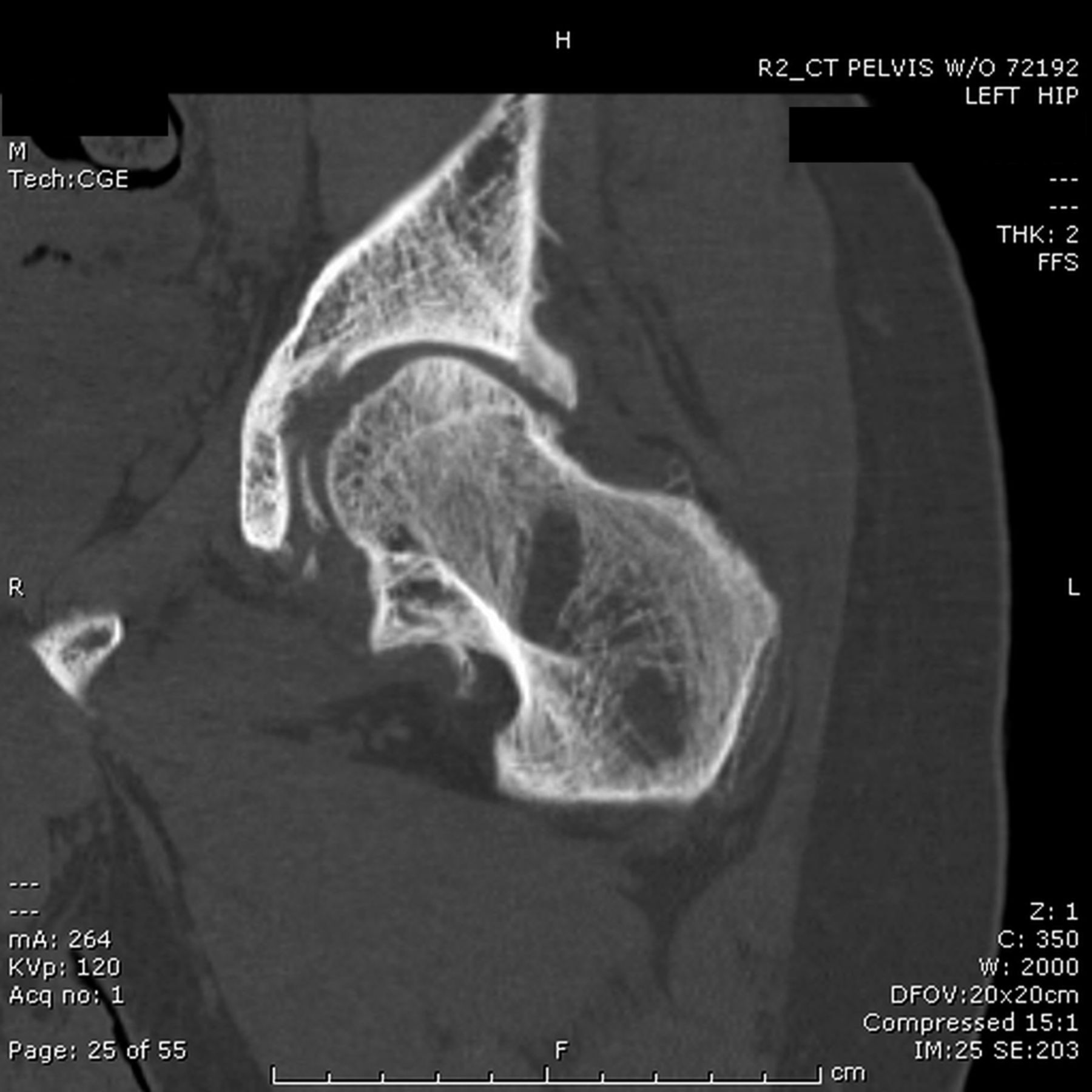
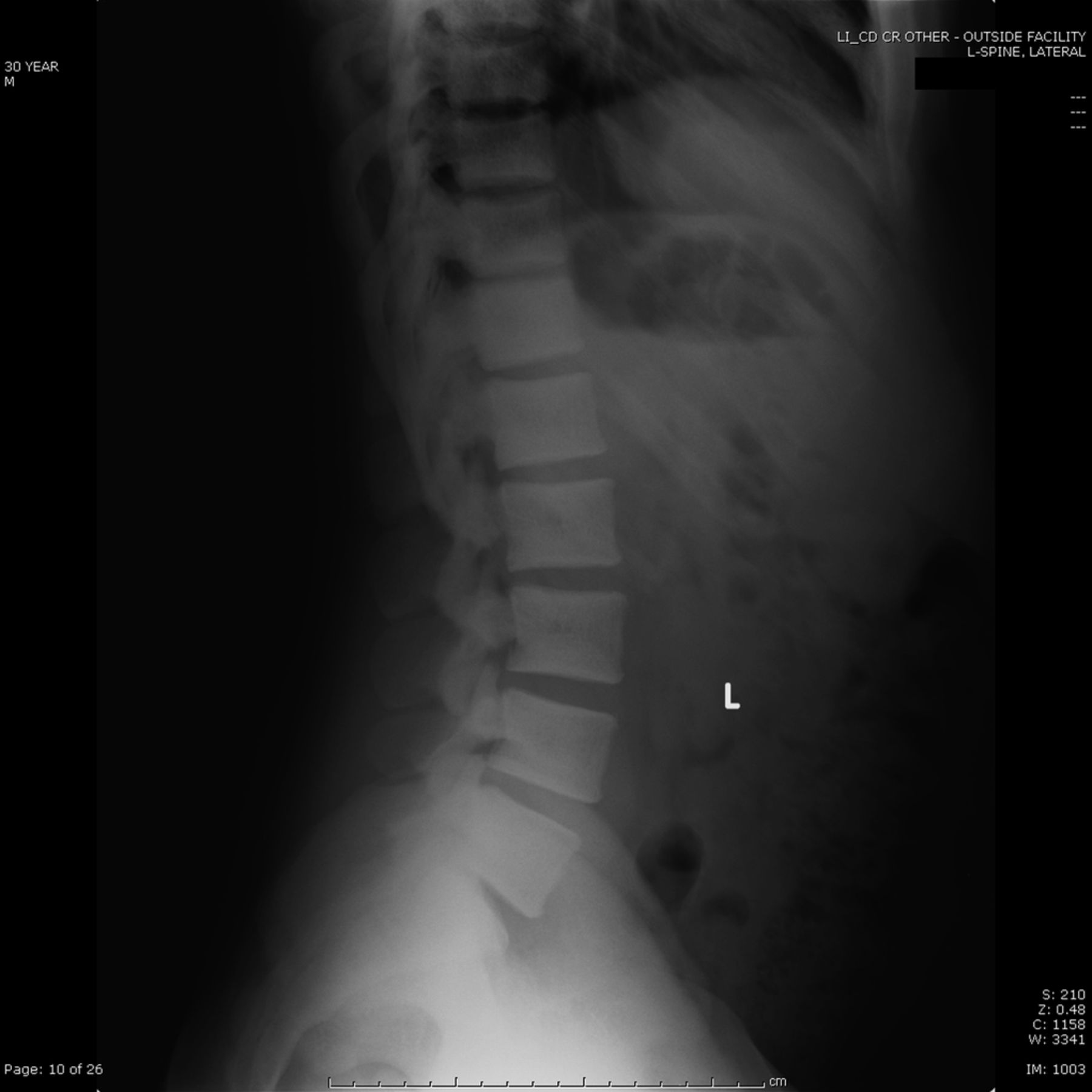
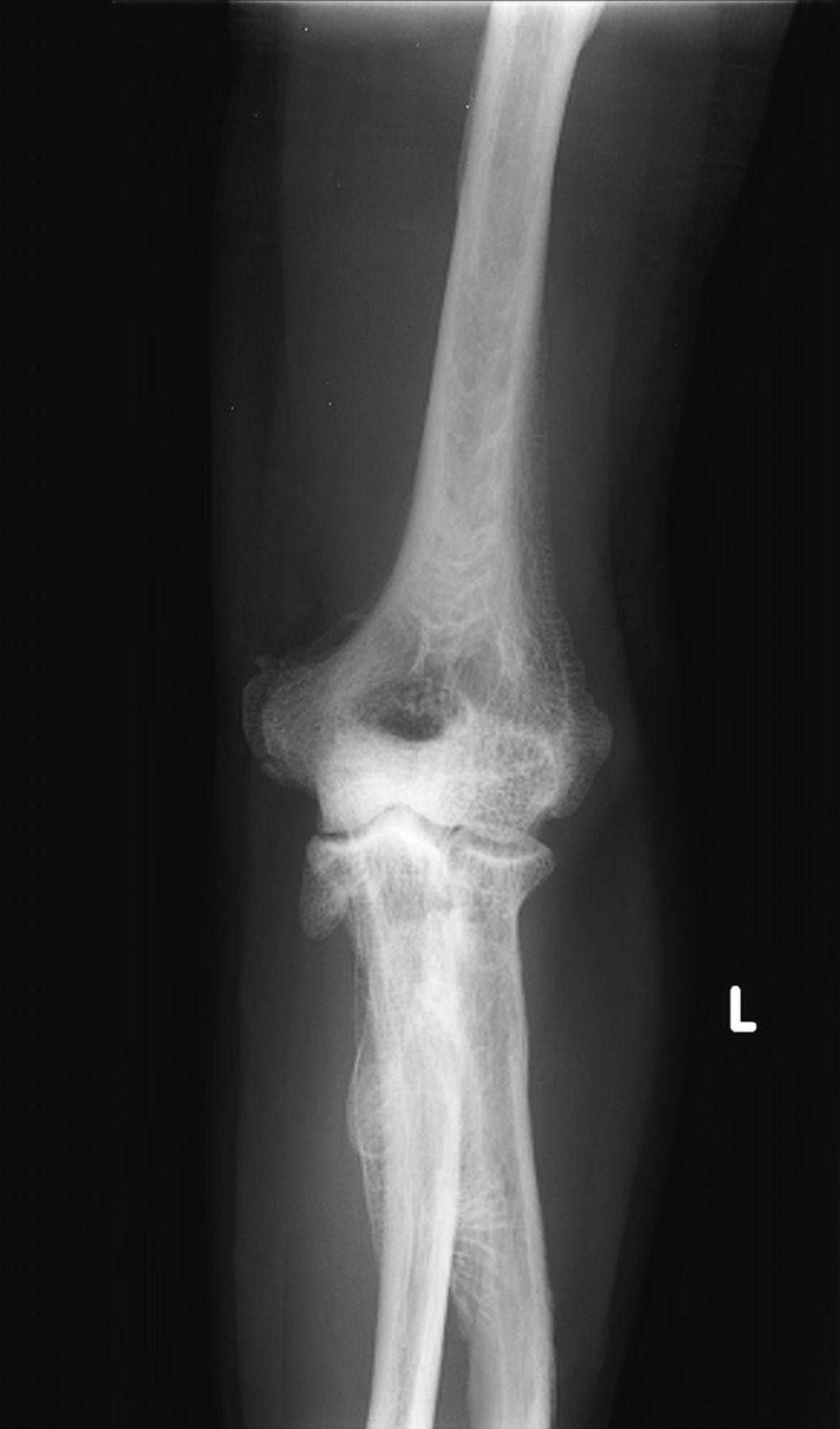
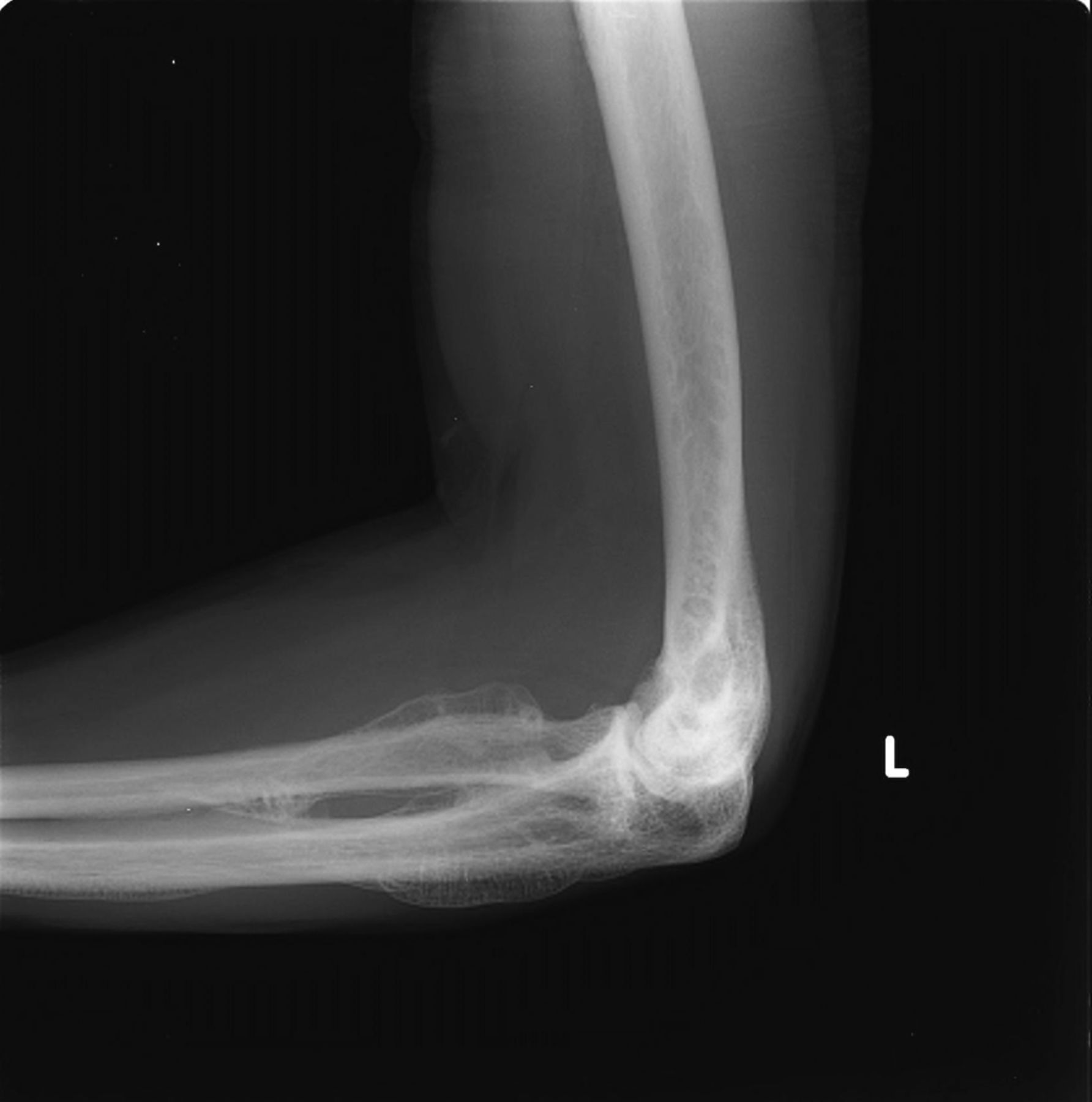
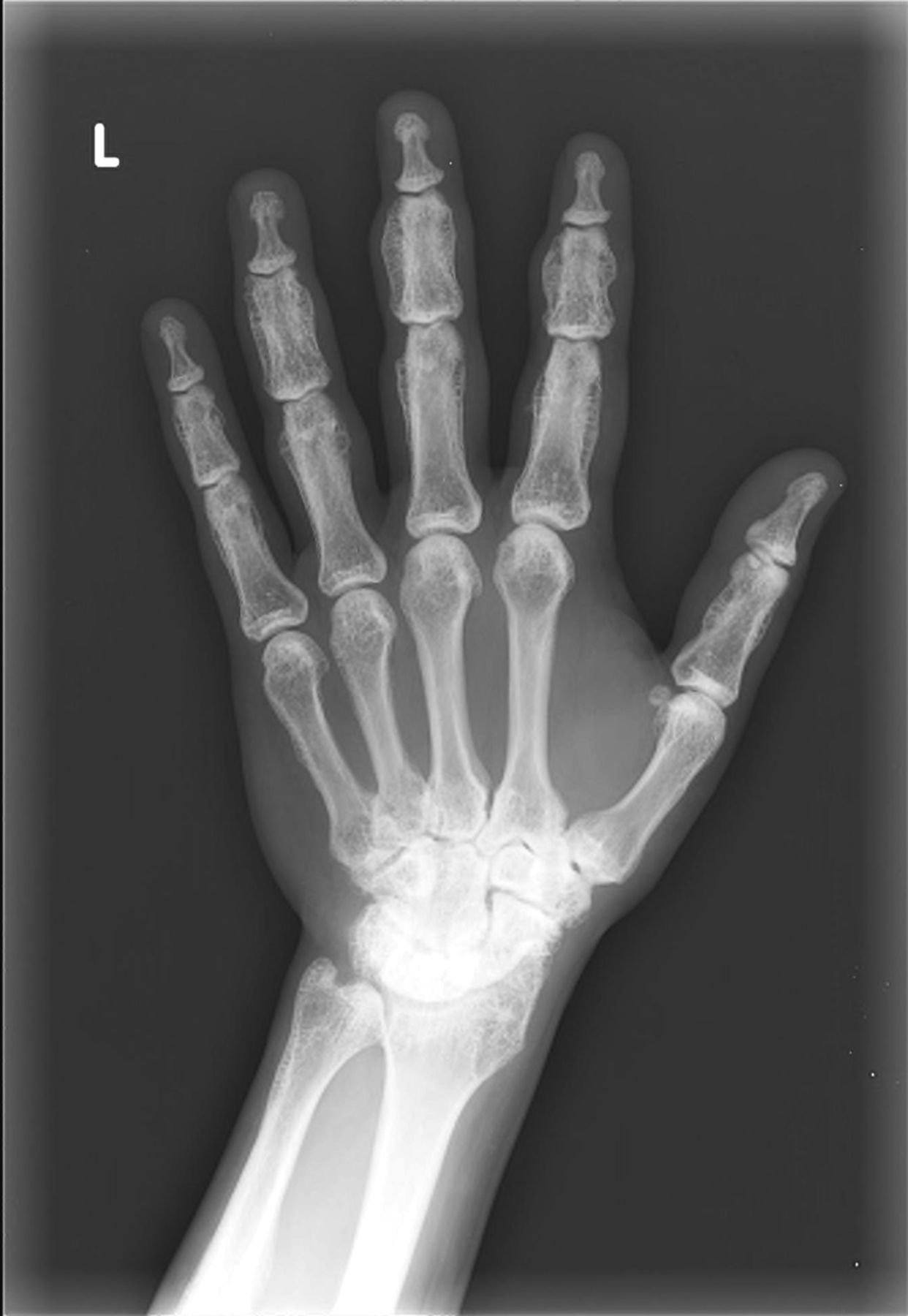
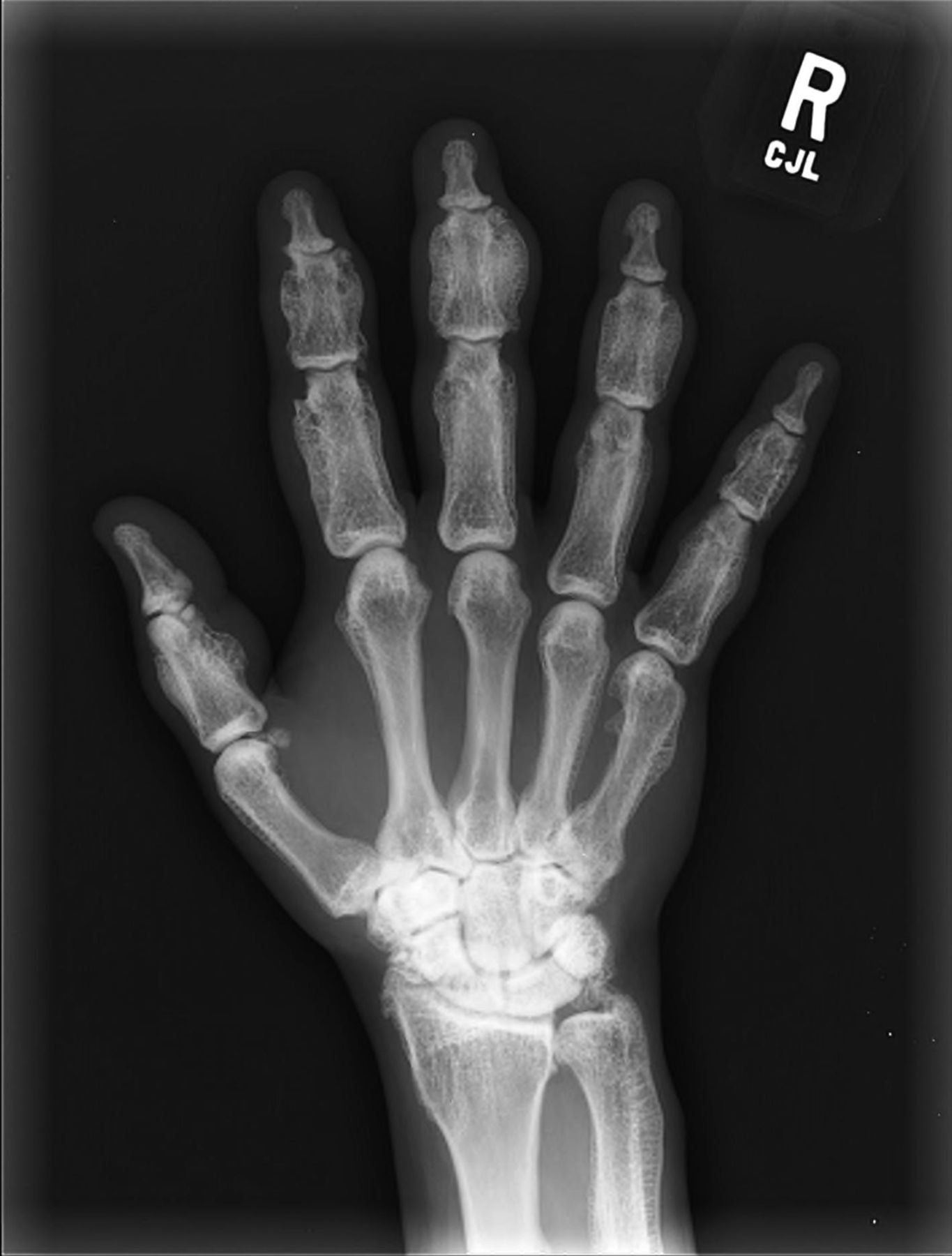

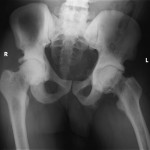 Fig. 1-A
Fig. 1-A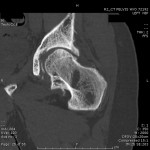 Fig. 1-B
Fig. 1-B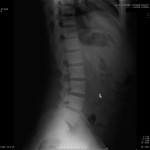 Fig. 2
Fig. 2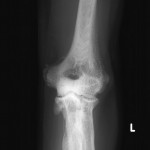 Fig. 3-A
Fig. 3-A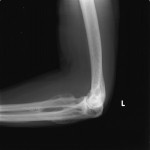 Fig. 3-B
Fig. 3-B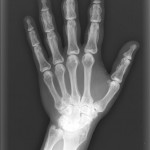 Fig. 4-A
Fig. 4-A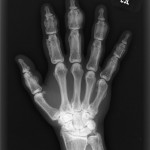 Fig. 4-B
Fig. 4-B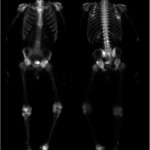 Fig. 5
Fig. 5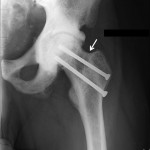 Fig. 6-A
Fig. 6-A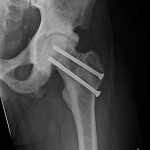 Fig. 6-B
Fig. 6-B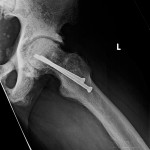 Fig. 6-C
Fig. 6-C