A Thirty-seven-Year-Old Man with a Painful Mass on the Left Knee
July 1, 2015
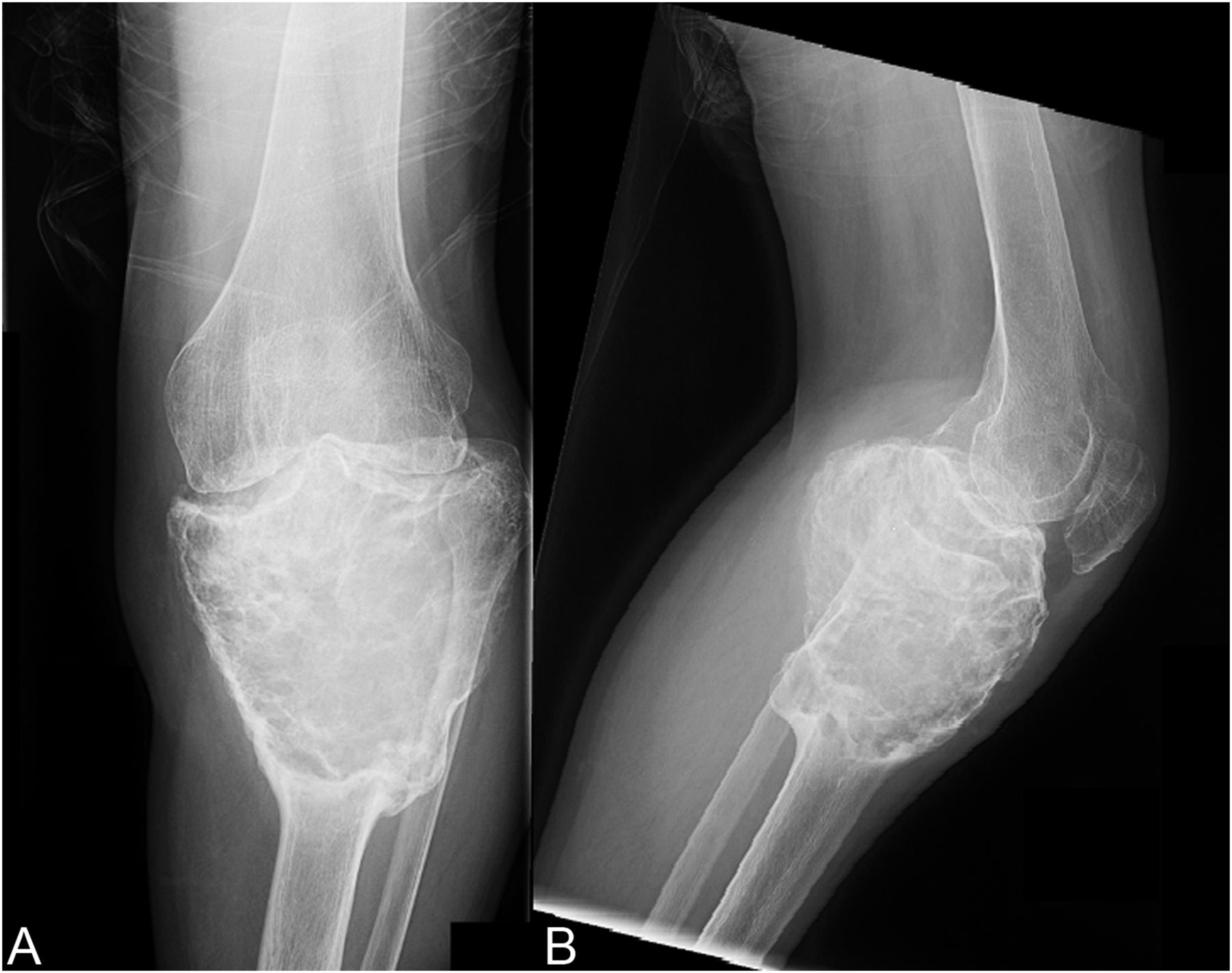
A thirty-seven-year-old man presented with a painful enlarging mass involving the left knee. He had first noted the mass one year previously, but did not seek immediate medical attention. He reported an increase in size and symptoms over the preceding six months, and ultimately sought care after developing progressive difficulty with walking. On physical examination, he had a large soft-tissue mass about the proximal part of the left tibia with limited knee motion. He was unable to bear weight because of the pain. Radiographs showed an expansile, lytic lesion involving the entire proximal part of the tibia (Figs. 1-A and 1-B). The computed tomographic (CT) and magnetic resonance imaging (MRI) scans showed an 11.9 × 7.4 × 10-cm lesion involving the entire proximal part of the tibia. The cortices were markedly thinned and expanded, and there was a large posterior soft-tissue component. A CT scan of the chest and a bone scan revealed no other focus of disease. All laboratory studies were normal. An open biopsy was performed (Fig. 2).
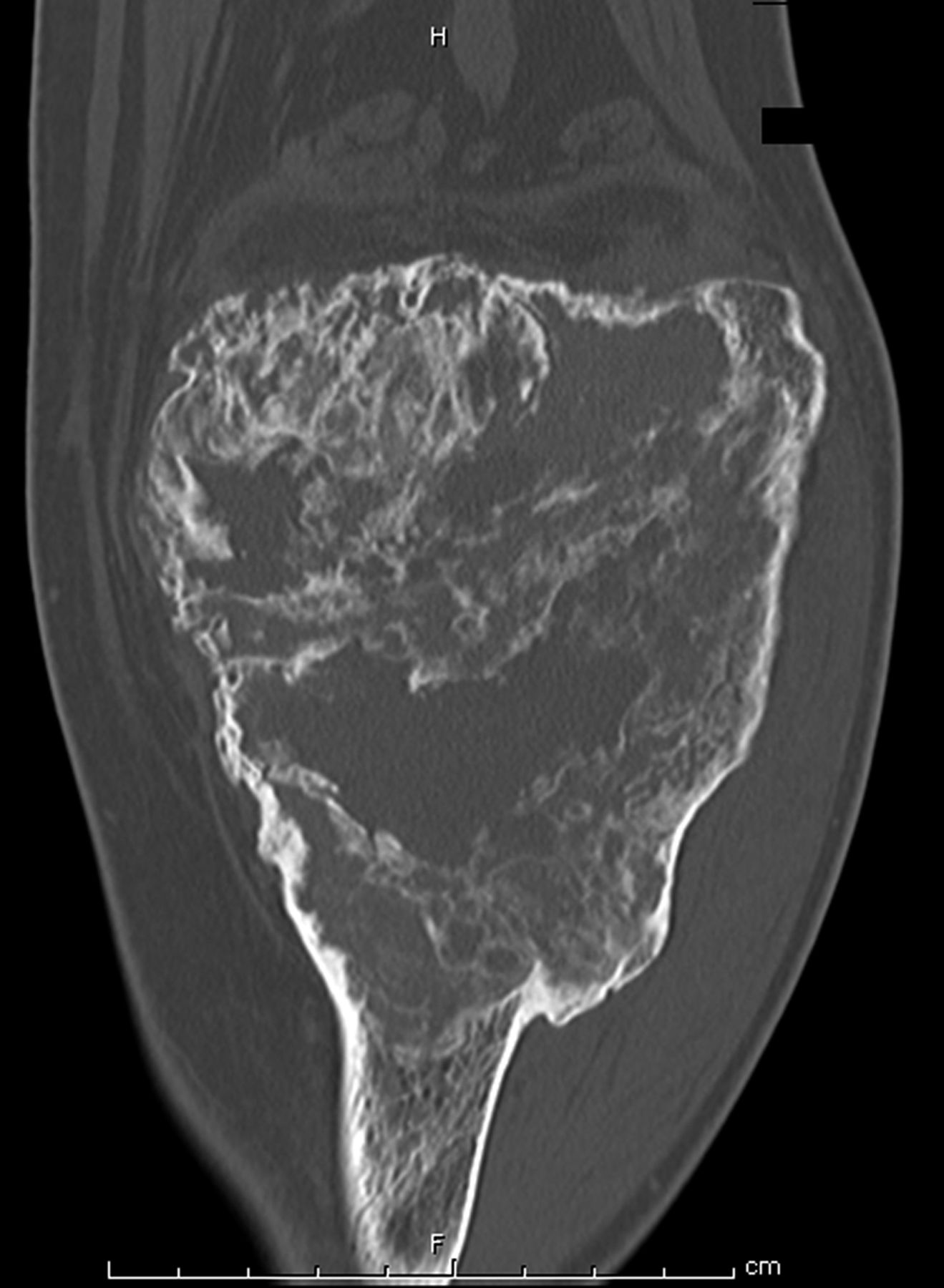
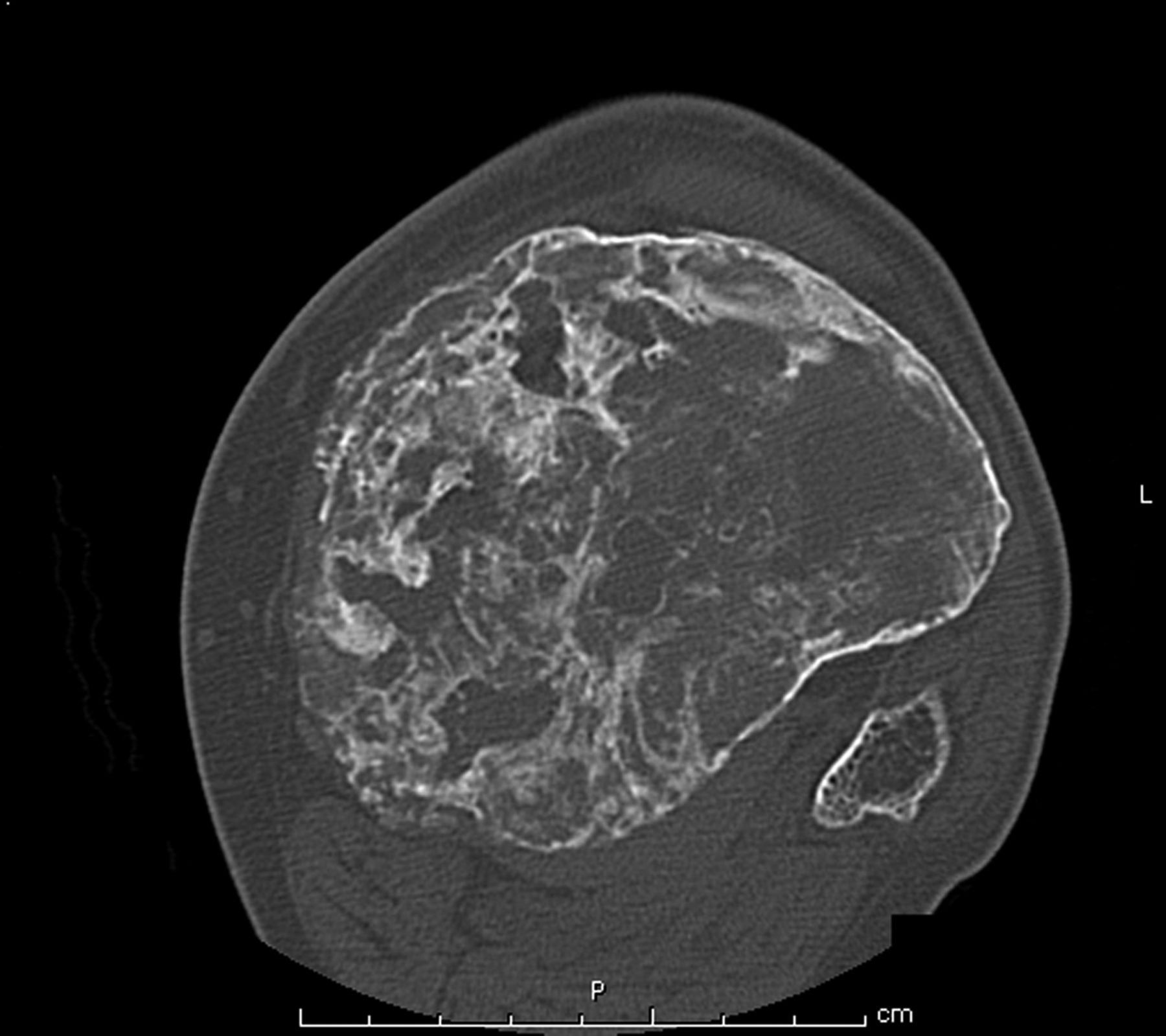
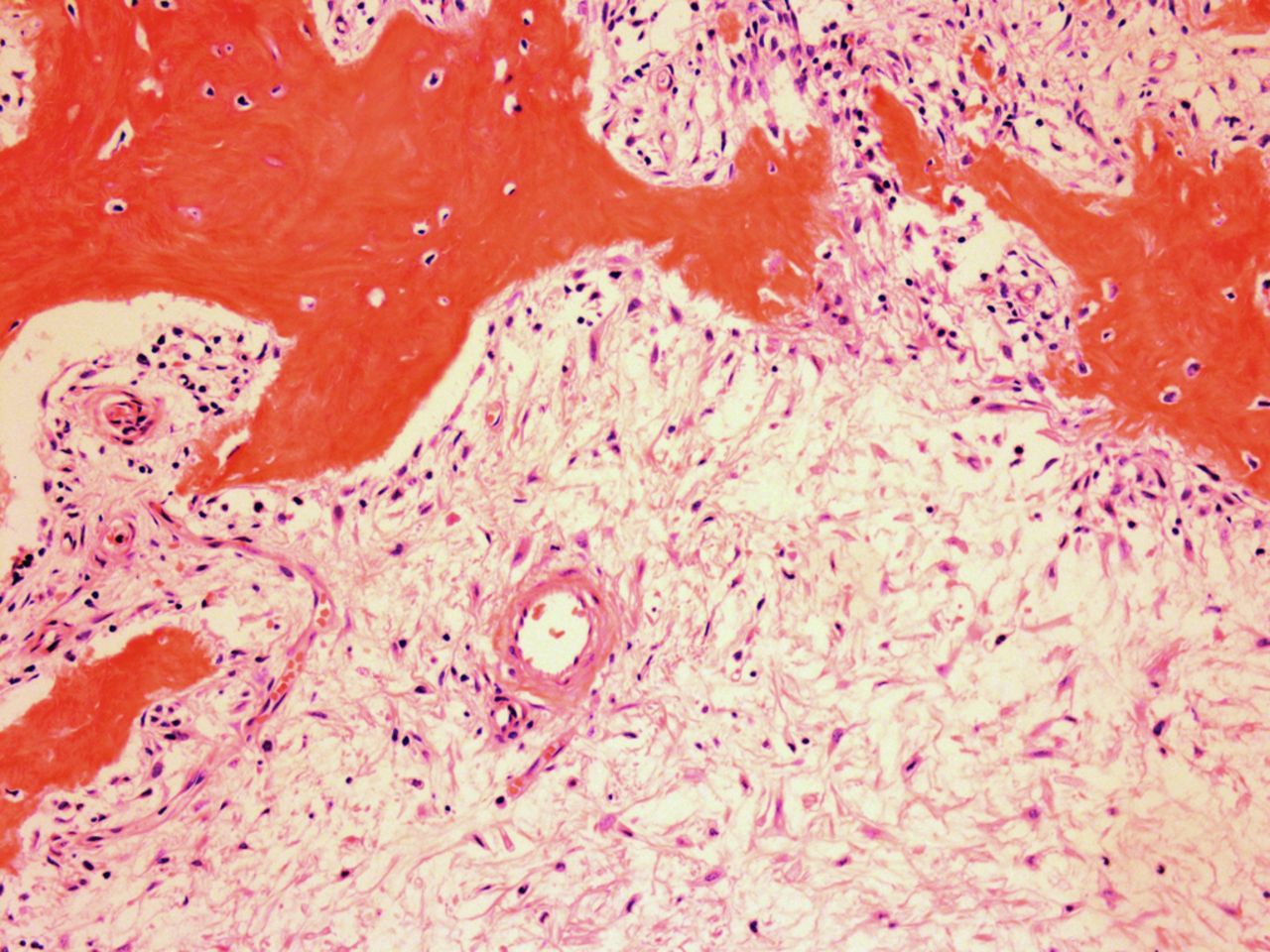
The patient was diagnosed with a giant cell tumor. The available management options were discussed with the patient; however, he was adamant that he did not wish to proceed with surgery. The patient was referred to a sarcoma treatment center, and he was enrolled in a phase-II clinical trial of denosumab for the treatment of locally advanced giant cell tumor of bone (ClinicalTrials.gov, Identifier NCT00680992). The patient was administered 120 mg of denosumab subcutaneously every four weeks, with a loading dose of 120 mg subcutaneously on study days eight and fifteen of the first month. Soon after starting therapy, the patient had a substantial reduction in pain and improvement in knee motion. Prior to treatment, the pain score was 9 on the 0 to 10 Numeric Pain Rating Scale; after two months of therapy, the pain was reported as 1. The treatment was well tolerated, with no reported toxicities, and after two months, we first began to appreciate a radiographic response. By twelve months, on radiographs, the cortices on the proximal part of the tibia had reconstituted, and the metaphysis and intramedullary regions were filling in with disorganized bone (Figs. 3-A and 3-B). A CT scan confirmed the radiographic findings (Figs. 4-A and 4-B). Because of the amount of new bone, the patient was able to progress to weight-bearing as tolerated with the use of crutches. After twelve months of treatment, we again discussed surgical options with the patient, including curettage, osteotomy, soft-tissue release, and bone-grafting of the proximal part of the tibia, or a resection and reconstruction with an endoprosthesis. The patient ultimately decided to undergo local excision of the tumor and reconstruction with a cemented modular rotating-hinge knee endoprosthesis for the proximal part of the tibia (Global Modular Replacement System; Stryker Orthopaedics, Rutherford, New Jersey). The specimen that was resected from the proximal part of the tibia measured 13.0 × 10.0 × 6.5 cm. Histological evaluation revealed (Fig. 5) rare scattered giant cells and prominent reactive bone formation replacing the tumor space. Tumor necrosis was greater than 95%. The margins were negative, and no vascular involvement was seen. The patient’s perioperative period was uneventful, and he left the hospital on the fourth postoperative day. At the most recent follow-up visit, six months after surgery, he had no clinical or radiographic evidence of local recurrence or distant spread of disease.
Proceed to Discussion >>Reference: Ahlmann ER, Wang L, Correa AJ, Fedenko AN. Near complete necrosis of a giant cell tumor after treatment with denosumab: a case report. JBJS Case Connect, 2012 Aug 08;2(3):e37.
The standard treatment for giant cell tumor of bone has been only surgical. However, with the recent discovery of key regulators of osteoclast formation and activity, including the receptor activators RANKL, RANK, and osteoprotegerin, a novel therapeutic option for the treatment of various skeletal disorders associated with extensive bone loss (including postmenopausal osteoporosis; multiple myeloma; metastatic bone disease; vascular calcification; genetic disorders of mineral metabolism, such as juvenile Paget disease; and, most recently, giant cell tumor of bone) has emerged. Side effects of denosumab have been reported to include nausea, arthralgia, respiratory tract infection, eczema, and osteonecrosis of the jaw. Histologically, giant cell tumors are characterized by large multinucleated giant cells that are phenotypically indistinguishable from osteoclasts located within sheets of neoplastic mononuclear stromal cells. These stromal cells are believed to induce bone resorption by recruiting osteoclast precursors and promoting their differentiation into functional osteoclasts. Experimental studies have demonstrated that RANKL is expressed by osteoblasts, whereas its receptor (RANK) is expressed in preosteoclasts. The interaction between RANKL and RANK stimulates osteoclast formation and differentiation by activation of several transcription factors. Denosumab is the human monoclonal antibody to RANKL, which has been shown to inhibit osteoclast-mediated bone destruction. Recently, a phase II multicenter clinical trial of the safety and efficacy of denosumab in patients with giant cell tumors of bone reported a response rate of 86%. Histological findings were available for twenty patients and showed near complete or complete elimination of giant cell tumors in all patients. Additionally, ten of the fifteen patients assessed by imaging studies had lack of tumor progression on imaging over a period of six months. Likewise, our patient demonstrated a remarkable radiologic response to denosumab. After twelve months of treatment, the bone had reconstituted to the point that the proximal part of the tibia was rimmed with cortical bone, and the metaphysis showed evidence of new bone formation. Although he was left with substantial joint space narrowing and a varus deformity because of the severity of the disease on initial presentation, he was able to fully bear weight without pain. Our patient had a remarkable histological response after treatment, including tumor necrosis greater than 95%, with replacement by a fibrous stroma and partially mineralized osteoid. It is highly unlikely that the tumor in this case underwent spontaneous necrosis to produce this result. Partial tumor necrosis has been reported infrequently within benign giant cell tumor of bone. Yao et al. reported a giant cell tumor of the distal part of the femur demonstrating extensive tumor necrosis; however, a viable region of giant cell tumor still remained. To the best of our knowledge, there is only one report in the literature that demonstrates complete spontaneous tumor necrosis in a previously untreated giant cell tumor of bone in a seventeen-year-old patient with a giant cell tumor of the distal part of the femur. However, neither of these two cases had had an initial biopsy showing an active giant cell tumor. In our case, it is more likely that the histological response was entirely attributable to treatment with denosumab. The fact that pretreatment with denosumab can produce extensive tumor necrosis may have prognostic implications. It remains to be seen whether neoadjuvant treatment with denosumab will decrease the recurrence rate of giant cell tumors, which has ranged from 8% to 27% despite the use of surgical adjuvants. Additional large-scale studies of denosumab as neoadjuvant treatment for giant cell tumors of bone are currently underway. Nevertheless, our case report confirms the therapeutic potential of denosumab to reverse progressive bone destruction and histologically produce almost complete elimination of giant cells in a patient with extremely advanced disease. Denosumab has shown early favorable results and may have the potential to change the management and outcome of this disease in the near future.
Reference: Ahlmann ER, Wang L, Correa AJ, Fedenko AN. Near complete necrosis of a giant cell tumor after treatment with denosumab: a case report. JBJS Case Connect, 2012 Aug 08;2(3):e37.
Paget disease of bone
Giant cell tumor
Chronic osteomyelitis
Metatstatic renal cell carcinoma
Brown tumor



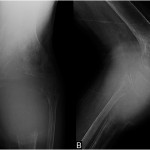 Fig. 1
Fig. 1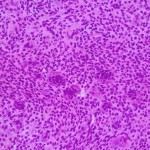 Fig. 2
Fig. 2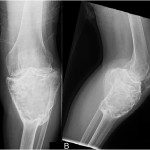 Fig. 3
Fig. 3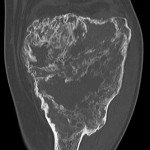 Fig. 4-A
Fig. 4-A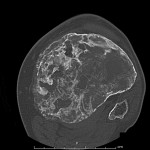 Fig. 4-B
Fig. 4-B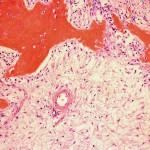 Fig. 5
Fig. 5