Upper-Extremity Neurovascular Compromise During Posterior Spinal Fusion for Idiopathic Scoliosis
January 5, 2011
A sixteen-year-old postmenarchal female with idiopathic scoliosis had a progressive 45° left lumbar curve and a 45° right thoracic curve that were unbalanced (Fig. 1-A). She was experiencing increasing lumbar back pain and reported having difficulties with balance. After discussion of the risks and benefits of operative compared with nonoperative treatment for idiopathic scoliosis, the patient and her family selected posterior spinal instrumentation and fusion treatment. In the operating room, general endotracheal anesthesia was induced. Intraoperative monitoring of somatosensory evoked potentials (SSEP) and motor evoked potentials commenced immediately after induction. Anesthesia was maintained with propofol and remifentanil intravenous infusions and low-dose sevoflurane (0.2 MAC [minimum alveolar concentration]). The maintenance anesthetic was adjusted and maintained at a constant rate prior to the onset of the critical surgical period. Invasive arterial pressure monitoring was instituted, and the mean arterial pressure was maintained at 65 to 75 mm Hg. Central venous pressure monitoring was not used. The patient was then turned prone and positioned in the routine manner on the Jackson spinal table. For positioning, the patient chest pad was placed at the chest level above the nipples and the hip pads were placed laterally just below the iliac crests. The abdomen was allowed to hang freely, without compression. The legs were supported in a sling. The shoulders were abducted 80°, with the elbow flexed and the arm positioned on a padded arm board. The head was placed in 7 pounds (3.18 kg) of traction through a head halter, and 7 pounds (3.18 kg) of skin traction was applied to each limb. The patient's height was 159.4 cm, and her weight was 62.3 kg. She had large breasts, which caused the chest roll to be positioned more cephalad than usual in order to allow the breasts to hang freely. A midline surgical approach to the posterior aspect of the spine was utilized. From the start of the procedure, the surgeons noted that blood loss seemed to be excessive from the superior portion of the wound, despite hypotensive anesthesia. Approximately three hours after induction of anesthesia, the anesthesia team noted intermittent dampening of the right-sided arterial waveform tracing, which responded to flushing of the arterial line. Noninvasive monitoring of blood-pressure measurements revealed no changes. Abnormal blanching was noted distal to the radial artery catheter with each flush of the arterial line. Prolonged capillary refill was also noted in the right upper extremity, and this condition did not respond to changes in arm positioning. Pulse oximetry values measured from the right hand were unchanged. Approximately one hour later, the anesthesia team noted that both hands had become cool, pale, and edematous and had prolonged capillary refill. The radial arterial tracing became difficult to maintain, and flushing produced marked finger blanching with slow capillary refill. No change in the blood pressure by noninvasive monitoring was noted other than a decrease of 5 mm Hg in the pulse pressure. The decreased pulse pressure was attributed to mild hypovolemia and treated accordingly with additional intravenous fluids. The mean arterial pressure measured with use of the arterial line remained stable, despite alterations in the systolic peak of the dampened waveform. At the same time, the amplitude of the motor evoked potentials decreased and latency significantly increased in both upper extremities, particularly on the right side. The evoked potentials in the lower extremity were unchanged. The patient was normocapnic, well oxygenated, and had a hemoglobin level of 10.4 g/dL (104 g/L) at the onset of the evoked potential changes. Increasing the arterial blood pressure had no effect on the amplitude or latency of the evoked potentials in the upper extremity. Neurovascular compression was suspected, and several attempts were made intraoperatively to improve the position of the patient and to reposition and repad the arms, but this did not change the physical findings. After discussion with the anesthesia team, it was decided to continue with placement of the instrumentation, and the procedure was completed as expeditiously as was safely possible. Sensory evoked potential recordings from the right arm did not improve or worsen throughout the remainder of the procedure. The estimated blood loss was 3100 mL, and the patient received a total of five units of packed red blood cells during the procedure. This blood loss and the resulting transfusion requirement were much higher than those typically encountered by us during idiopathic scoliosis procedures. The duration of surgery was seven hours and five minutes. At the end of the procedure, when the patient was turned supine, it was noted that both hands were very white, cold, and swollen. There was a clear demarcation between the white hands and normally perfused tissue at the level of the distal aspect of the forearm. Her face was also swollen initially, but not more than would be expected following a lengthy prone procedure. She was extubated without difficulty. When she awoke, a motor and sensory examination was performed, the results of which were completely normal. Over a period of forty-five minutes, the blanching in the arms subsided and it became clear that the hands were perfused, although still cool. Her hands did remain unusually cool and pale for several days after surgery. She was monitored overnight in the pediatric intensive care unit and then transferred to the pediatric ward. The postoperative course was uneventful, and the patient was discharged home on the fifth postoperative day. At the one-year follow-up visit, she reported that the hands and feet would intermittently become bright red and cold for about thirty minutes; however, she had had no episodes of the hands or feet turning white. The one-year postoperative radiograph is shown in Figure 1-B.
In the immediate postoperative period, in an attempt to determine the etiology of the intraoperative findings, chest computed tomography with contrast was acquired with the patient supine. A second scan was acquired two weeks postoperatively, when the patient was able to lie comfortably prone within the computed tomography gantry. During the computed tomography scan in which the patient was positioned prone, the patient chest pad on the Jackson table was positioned by the operating surgeon as it had been during the operative procedure. The computed tomography images, acquired immediately thereafter, demonstrated unambiguous compression of the superior vena cava. There was also a clear reduction in the distance from the anterior aspect of the aortic arch to the posterior aspect of the sternum (Figs. 2-A and 2-B). The clinical findings of upper-extremity pallor and swelling were consistent with the clinical diagnosis of phlegmasia alba dolens, most likely caused by intraoperative superior vena cava compression from the chest pad that was used during positioning. In this condition, capillary hypoperfusion develops from the increased tissue pressure, which in turn is a result of increased venous pressure. As the hands are most distal and dependent during surgery, the effect of the increased tissue pressure becomes most evident there, accounting for the sharp line of demarcation noted in the presence of phlegmasia alba.
Proceed to Discussion >>Reference: Hogan KA, Harvey SC, Conway WF, DeRosimo JF, Gross RH. Superior vena cava compression during posterior spinal fusion for idiopathic scoliosis. A case report. J Bone Joint Surg Am. 2009;91:696-700.
Correctly positioning a patient prior to beginning any surgical procedure requires careful attention to padding the osseous prominences and ensuring that excessive pressure or stretch is not placed on the neurovascular structures. Prone positioning for spinal fusion poses a unique set of challenges for both the surgeon and the anesthesiologist. The airway must be secure. Prone positioning has been associated with visual compromise related to increased ocular pressure, arterial occlusion, and hypoxia. The shoulders should be positioned in <90° of abduction to avoid stretching the brachial plexus, and some authors recommend that the arms be tucked at the sides. The elbows should be padded to prevent external compression on the ulnar nerve, and extreme flexion of the elbow may increase the risk of ulnar nerve injury. The lateral femoral cutaneous nerve can be compressed at the anterior superior iliac spine by bolsters when the patient is in the prone position. The abdomen should hang freely to decrease intra-abdominal pressure and prevent an increase in blood loss as a result of venous hypertension. Despite the best efforts of surgeons and anesthesiologists, complications related to positioning do still occur. Intraoperative changes in SSEP recordings may indicate early neurologic compromise related to positioning. In one study of 1000 spinal fusions for scoliosis, there was a 6.1% prevalence of changes in upper-extremity SSEP recordings that resolved with changes in arm positioning. Vossler and colleagues also reported on a case of lower-extremity ischemia that was detected by the loss of intraoperative SSEPs and that resolved with a change in patient positioning. Loss of arterial blood-pressure tracings has also been associated with positioning complications, such as shoulder dislocation. The use of pulse oximetry has also been advocated as a method of obtaining early intraoperative warning of inadequate perfusion to the limbs. One of us (R.H.G.), after visiting Professor Dubousset, prefers to use traction on the lower extremities and head during all posterior spinal procedures. The advantages described by Dubousset are a decrease in postoperative decompensation and a gradual correction of deformity during the dissection of soft tissue, including removal of all interspinous ligaments and joint capsules. To our knowledge, this technique was used in just one published series in the English-language literature, and those authors did not describe any improvement in alignment with use of this type of intraoperative traction. We do not believe that use of traction had any effect on the complication reported here. During our patient's procedure, changes in both arterial blood-pressure tracings and SSEP recordings alerted the anesthesiologists and surgeons to the presence of compression to the upper limbs. This particular case is unique because, to our knowledge, it is the first report of intraoperative superior vena cava compression caused by prone positioning for spinal surgery. The clinical presentation of white, swollen upper extremities is similar to that of phlegmasia alba dolens, a condition associated with massive thrombosis of the large veins. It occurs most commonly in the setting of malignant disease but is also associated with hypercoagulable syndromes and pregnancy. External causes of superior vena cava compression include malignant neoplasm, mediastinitis, goiter, teratoma, and aneurysm. Flow can also be disrupted internally by massive thrombosis. The superior vena cava is relatively thin walled and is more easily compressible than the aorta. Compression of the superior vena cava has not previously been reported to be caused by prone positioning for surgery, and surgeons treating scoliosis may not be familiar with this entity. Phlegmasia alba dolens is more commonly seen in the lower extremities. The pathophysiology of this condition is the elevation of interstitial pressure of the tissue secondary to venous congestion. When the interstitial pressure of the tissue exceeds the capillary perfusion pressure, hypoperfusion occurs. Upper-extremity involvement is relatively rare and is typically associated with severe medical comorbidities. Complete venous occlusion may lead to phlegmasia cerulea dolens. This condition occurs when tissue ischemia leads to the buildup of deoxygenated hemoglobin, producing cyanotic color changes in the extremity. With further progression, ulceration and venous gangrene can occur. In our patient, the clinical picture was consistent with phlegmasia alba dolens. The computed tomography scans that were acquired with our patient prone on the Jackson chest pad clearly show narrowing of the superior vena cava immediately after the patient was placed in that position. With prolonged positioning during a posterior spinal fusion, it appears that the force of her body weight against the bolster resulted in compression of the superior vena cava sufficient to cause clinical signs during surgery, including the excessive blood loss from the proximal portion of the operative exposure. The obstruction of the superior vena cava was not complete, and the symptoms largely resolved after the patient was placed in the supine position. In conclusion, the importance of attention to detail when positioning a patient for a surgical procedure cannot be overemphasized. Changes in pulse oximetry waveforms, arterial waveforms, and SSEP recordings may indicate early problems from external compression. If it can be done safely, an attempt should be made to reposition the patient during the procedure. Finally, a patient's body habitus is a factor in positioning as well. Consideration should be made for alternative positioning if the breasts or abdomen do not hang freely or if they compromise positioning. Finally, the Jackson spinal table with the chest pad may not be suitable for all patients undergoing surgery to correct spinal deformity, and alternate methods with lateral pads may be preferable.
Reference: Hogan KA, Harvey SC, Conway WF, DeRosimo JF, Gross RH. Superior vena cava compression during posterior spinal fusion for idiopathic scoliosis. A case report. J Bone Joint Surg Am. 2009;91:696-700.
Intraoperative head and lower-extremity traction with hypotensive anesthesia
Compression of the superior vena cava
Compression of the brachial plexus and subclavian vessels
Improper position of central venous catheter

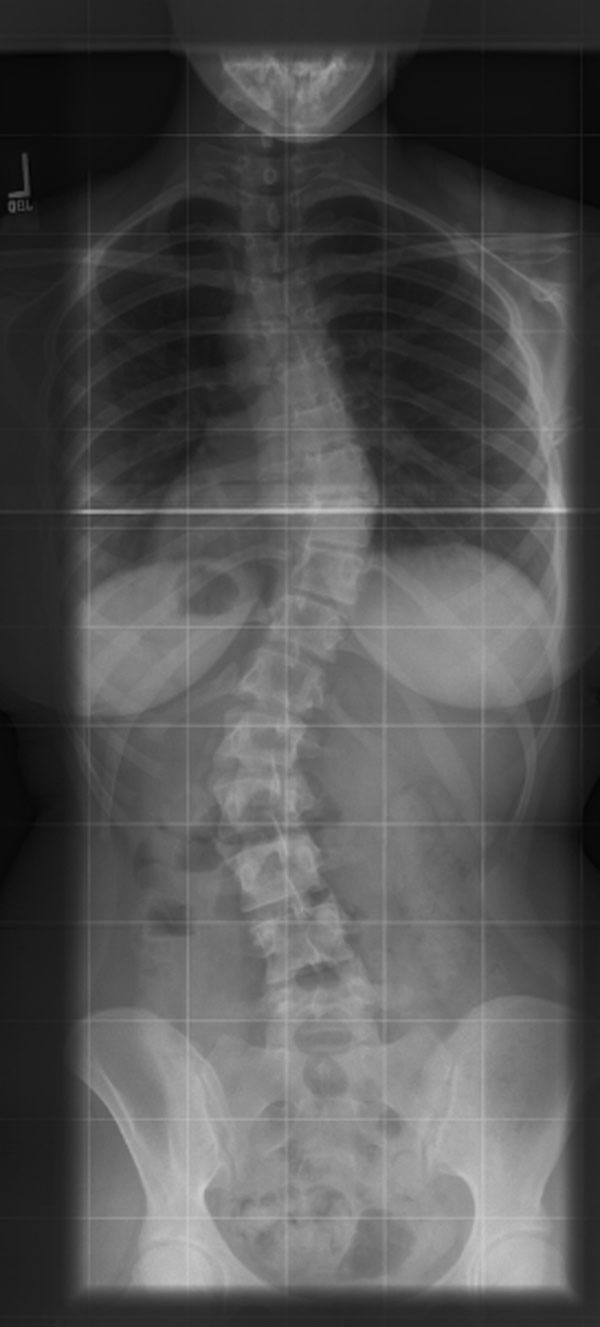
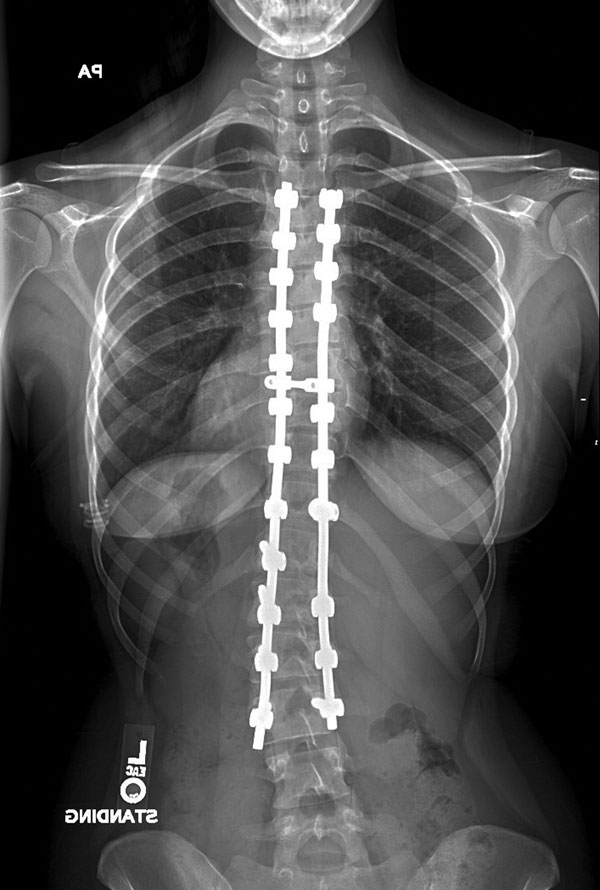
 Fig. 1-A
Fig. 1-A Fig. 1-B
Fig. 1-B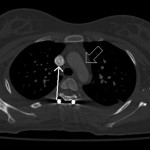 Fig. 2-A
Fig. 2-A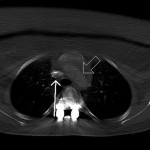 Fig. 2-B
Fig. 2-B
