A Thirty-nine-Year-Old Man with Sharp, Persistent, and Constant Midline Posterior Thoracic Pain
January 1, 2014
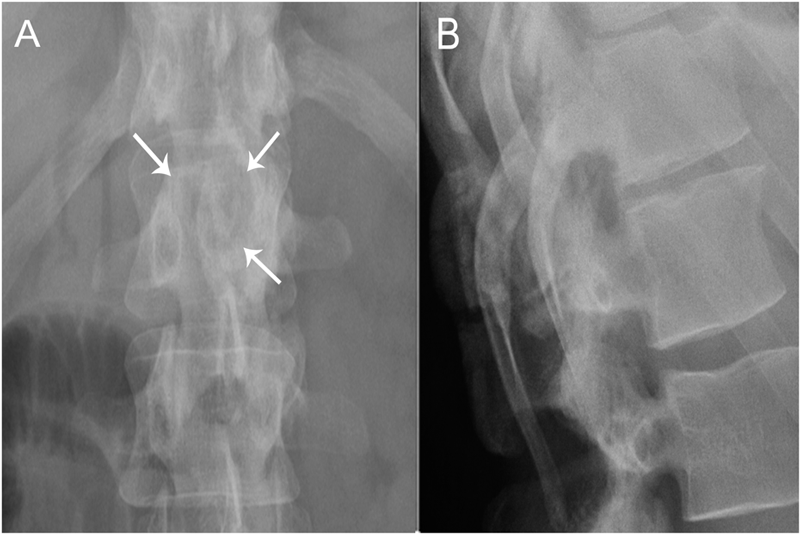
A thirty-nine-year-old man presented with symptoms of sharp, persistent, and constant midline posterior thoracic pain of insidious onset and moderate strength that sometimes awakened him from sleep but did not radiate. The pain was aggravated by weight-bearing, lifting, and movement; it was partially alleviated with over-the-counter pain medications. By the patient’s estimation, it overlapped in nature and severity with some residual pain from a previous, and mostly thought to be irrelevant, lifting injury two years before, for which he had sought chiropractic assistance and had experienced temporary relief. No radiographs had been obtained at that time. The pain was not associated with any neurological disturbance. Past medical and surgical history and review of systems were unrevealing. Specifically, there was no history of previously identified bone lesions or malignancy, and he had never undergone any spine procedures. The physical examination was unremarkable except for localized tenderness to palpation and percussion over the T12 and L1 region, some prominence at the T12 spinous process, and increased pain at 90° of flexion. Otherwise, the patient had full active range of spine motion. The overlying skin was unremarkable. Neurological function was intact in both lower extremities, including good heel-and-toe walking. We obtained multiple imaging studies. The radiographs showed a lesion involving the spinous process of T12 (Figs. 1-A and 1-B). The magnetic resonance imaging (MRI) scan revealed a 3.6 × 3.1 × 2.8-cm well-marginated uniformly enhancing lesion within the spinous process of T12, extending to both laminae and the spinal canal, causing mild cord impingement posteriorly (Figs. 2-A through 2-D). The computed tomography (CT) scan showed a 3.7 × 3.1 × 2.9-cm well-defined lytic lesion in the spinous process of T12 surrounded by sclerotic margin, containing some osseous intralesional matrix, and showing some encroachment of the spinal canal (Figs. 3-A, 3-B, and 3-C). The technetium-99m whole-body scan showed a solitary site of increased activity posteriorly at T12 (Fig. 4).
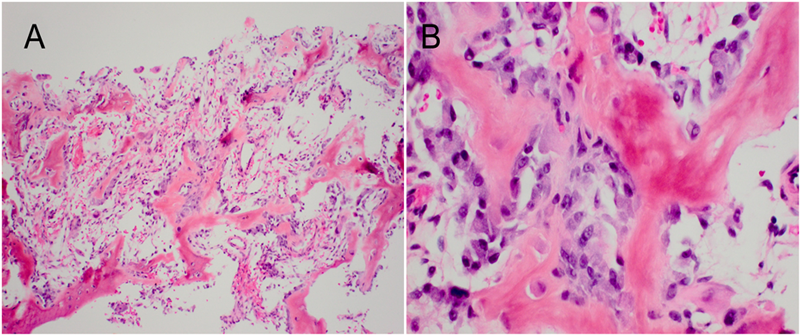
Based upon the location within the posterior elements, the associated pain, the size of the lesion (with a nidus larger than 2 cm), and the CT evidence of matrix ossification, the working clinical diagnosis was osteoblastoma. In order to confirm this clinical suspicion prior to definitive treatment, a CT-guided needle biopsy was performed by interventional radiology. The histology sections were comprised of woven bone with diffuse, plump, benign-appearing osteoblastic rimming and scattered osteoclasts separated by a highly vascular stroma. The stroma in the intertrabecular spaces consisted of loose connective tissue with no evidence of spindle-cell proliferation, atypia, or atypical mitosis (Figs. 5-A and 5-B). The pathological findings confirmed the diagnosis of osteoblastoma of the T12 spinous process. The patient was then referred to the spine specialist in our facility for definitive surgical intervention. En bloc resection of the tumor was planned. However, the patient canceled the procedure and did not return for immediate follow-up. Five years and four months after the initial presentation, the patient returned with symptoms of unresolved and constant posterior lower thoracic nonradiating pain without any neurological effect. He justified waiting this long because of a work-related financial problem regarding insurance coverage. The musculoskeletal examination revealed a prominence over the T12 spinous process that was tender to percussion. Again, he had full range of motion of the spine. He was neurologically intact. Repeat radiographs (Figs. 6-A and 6-B) and CT scans (Fig. 7) revealed no progression in the tumor size; only increasing peripheral sclerosis was present. The patient was again referred to our spine specialist to be reassessed and to discuss treatment options. He continued ongoing consultation about the recommended surgical treatment, but did not commit to a surgical procedure.
Proceed to Discussion >>Reference: Elhawi ME, De La Roza GL, Damron TA. Natural history of untreated osteoblastoma: a case report. JBJS Case Connector. 2013 Nov 13;3(4):e110.
Osteoblastoma is a rare bone-forming tumor that represents no more than 1% of all bone tumors. The bones of the spine, femur, tibia, and mandible constitute 75% of all osteoblastomas. The spine is most commonly affected; osteoblastoma of the cervical spine occurs more often than osteoblastoma of the lumbar spine. For spinal osteoblastoma, the posterior elements are most commonly involved, although primary involvement of the vertebral body accounts for 3%. Osteoblastoma is a tumor of adolescence and early adulthood, with 90% of all lesions occurring before age thirty. Sex predilection favors involvement in males over females in a 2 to 2.5:1 ratio. Patients with osteoblastoma typically present with local or radicular pain. Radiographs show a rounded to oval well-circumscribed osteolytic lesion with a variable degree of surrounding sclerosis. Histologically, osteoblastoma is characterized by a highly vascular stroma traversed by irregular trabeculae of woven bone that is lined by a variable number of benign-appearing osteoblasts. Although osteoblastomas are typically best characterized as “active” lesions, according to the classification of Enneking and the Musculoskeletal Tumor Society, they have the propensity for more aggressive behavior, bordering on malignancy. Hence, for the vast majority of osteoblastomas, the recommended treatment is surgical, whether by en bloc resection or extended intralesional curettage. Our patient provides a unique opportunity to present the natural history of an untreated osteoblastoma that did not demonstrate the more typical aggressiveness of this tumor, thus underscoring the broad range of behavior of osteoblastomas. The more aggressive behavior of osteoblastoma is one of the features that distinguishes it from its nonprogressive counterpart, osteoid osteoma. While osteoid osteoma has a central radiolucency similar to osteoblastoma, the size of its central nidus is less than 1.5 to 2 cm in diameter and typically is surrounded by a thick rind of reactive bone in all but the juxta-articular cases. Osteoblastoma is also distinct from osteoid osteoma in that it lacks the classic nocturnal pain that is relieved so impressively and completely by nonsteroidal anti-inflammatory medications in 70% of patients with osteoid osteomas. Because of their frequent spinal location and propensity for enlargement, osteoblastomas are much more likely to cause neurological disturbances than osteoid osteomas. Because they behave distinctively, osteoblastomas and osteoid osteomas are treated differently. Currently, most osteoid osteomas are treated successfully with radiofrequency ablation, although they may also be managed with nonsteroidal anti-inflammatory medications to control the pain until the symptoms abate. Radiofrequency ablation is 95% successful after the first treatment. The lack of radiographic progression of even untreated osteoid osteomas is well documented. By contrast, the recommended treatment for osteoblastoma is either extended curettage or en bloc resection; even after this more aggressive treatment, the local recurrence rate is up to 20%. The osteoblastoma behavioral spectrum ranges from the simply “active” lesion, characterized by pain and progressive but slow enlargement, to the “aggressive” lesion, typified by cortical destruction and soft-tissue extension. In some cases, these more aggressive osteoblastomas have been characterized histologically by epithelioid osteoblasts and higher mitotic activity than the classic benign forms. The information on the natural history of osteoblastomas is primarily inferred from the radiographic appearance at the time of presentation because, in most cases, surgery is recommended and performed within a short period of days to weeks. To the best of our knowledge, there have been very few published true natural history cases of observed, untreated osteoblastomas. Emmez et al. reported a case of lumbar osteoblastoma that increased only 0.5 cm in diameter over two years of documented radiographic investigations, with eight years of clinical history and three years of clinical observation. In two cases of untreated sacral lesions from another study, only one had a comparative radiograph published, which showed no increase in tumor size over a twelve-year follow-up period. However, the focus of that report was on how the two patients had remained asymptomatic over twelve and seven years of clinical follow-up, respectively; the second patient did not have comparative serial imaging. In our patient, the tumor’s size remained unchanged over a period of five years and four months, which demonstrates the variability in the behavior of this type of tumor. Unfortunately, to our knowledge, there are very few cases that have observed the natural history of an osteoblastoma. This renders it difficult to predict common identifying features for the stable cases. Accordingly, despite our patient’s stable condition, we do not suggest changing the current recommended surgical treatment for osteoblastoma.
Reference: Elhawi ME, De La Roza GL, Damron TA. Natural history of untreated osteoblastoma: a case report. JBJS Case Connector. 2013 Nov 13;3(4):e110.
Osteoid osteoma involving the spinous process of T12
Aneurysmal bone cyst of T12
Fracture of the posterior elements of T12
Osteoblastoma involving T12
Hemangioma of the posterior elements of T12

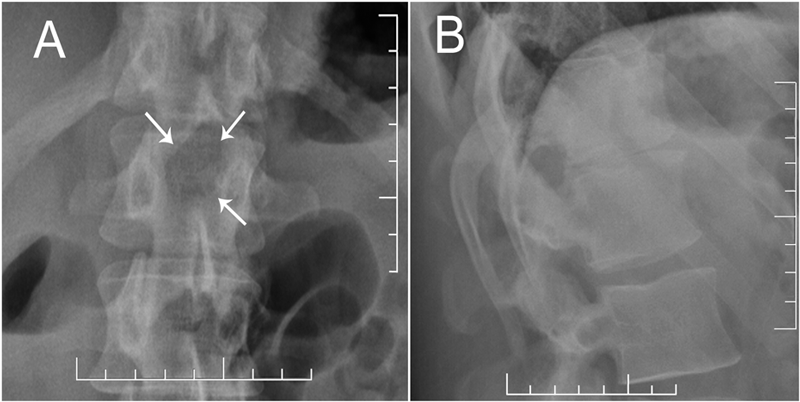
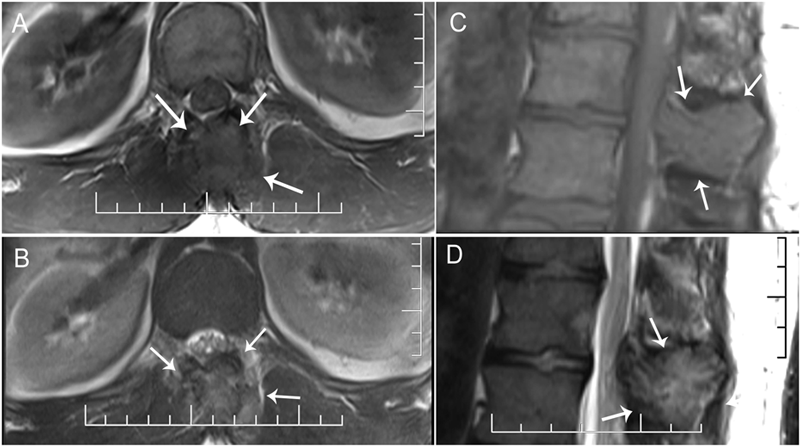
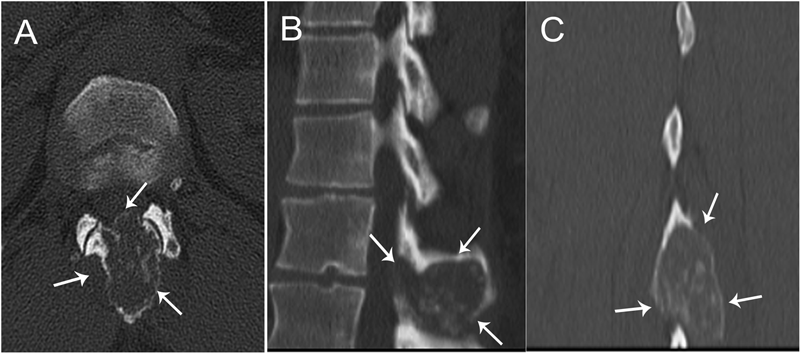
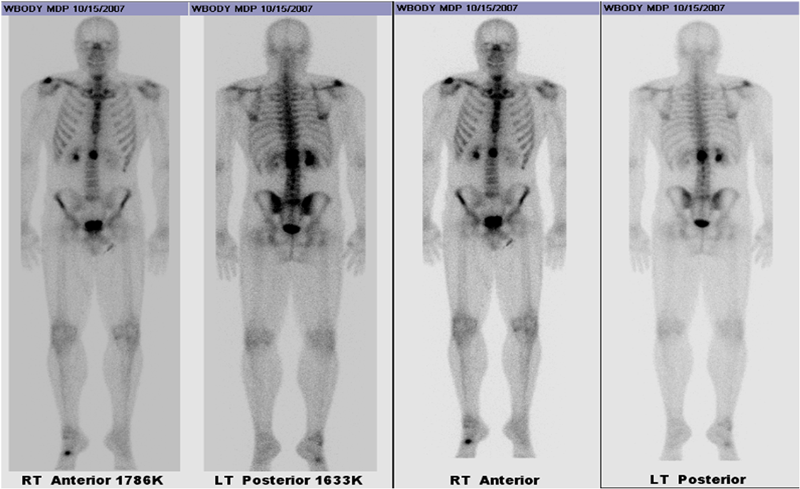
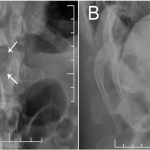 Fig. 1
Fig. 1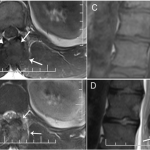 Fig. 2
Fig. 2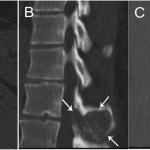 Fig. 3
Fig. 3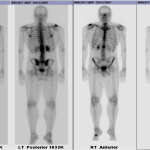 Fig. 4
Fig. 4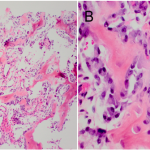 Fig. 5
Fig. 5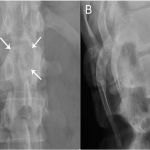 Fig. 6
Fig. 6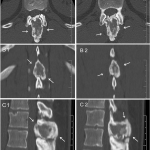 Fig. 7
Fig. 7