A Three-Month-Old Boy with Upper-Extremity Amyoplasia
July 18, 2012
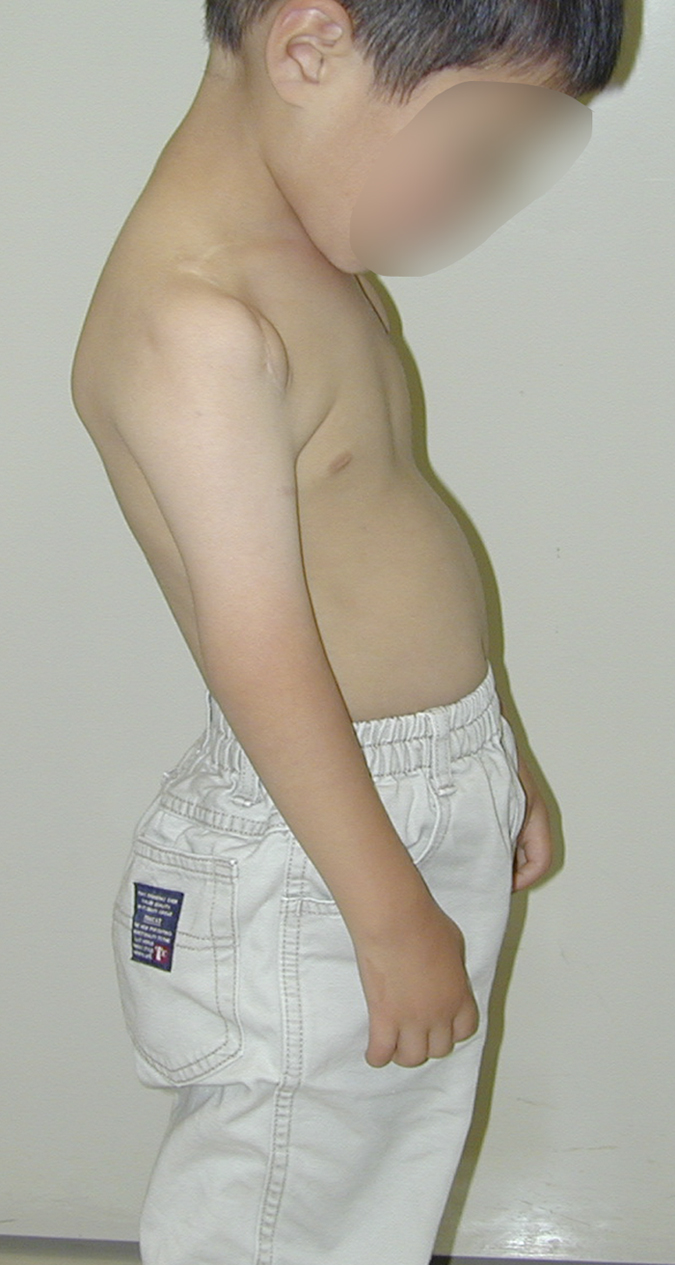
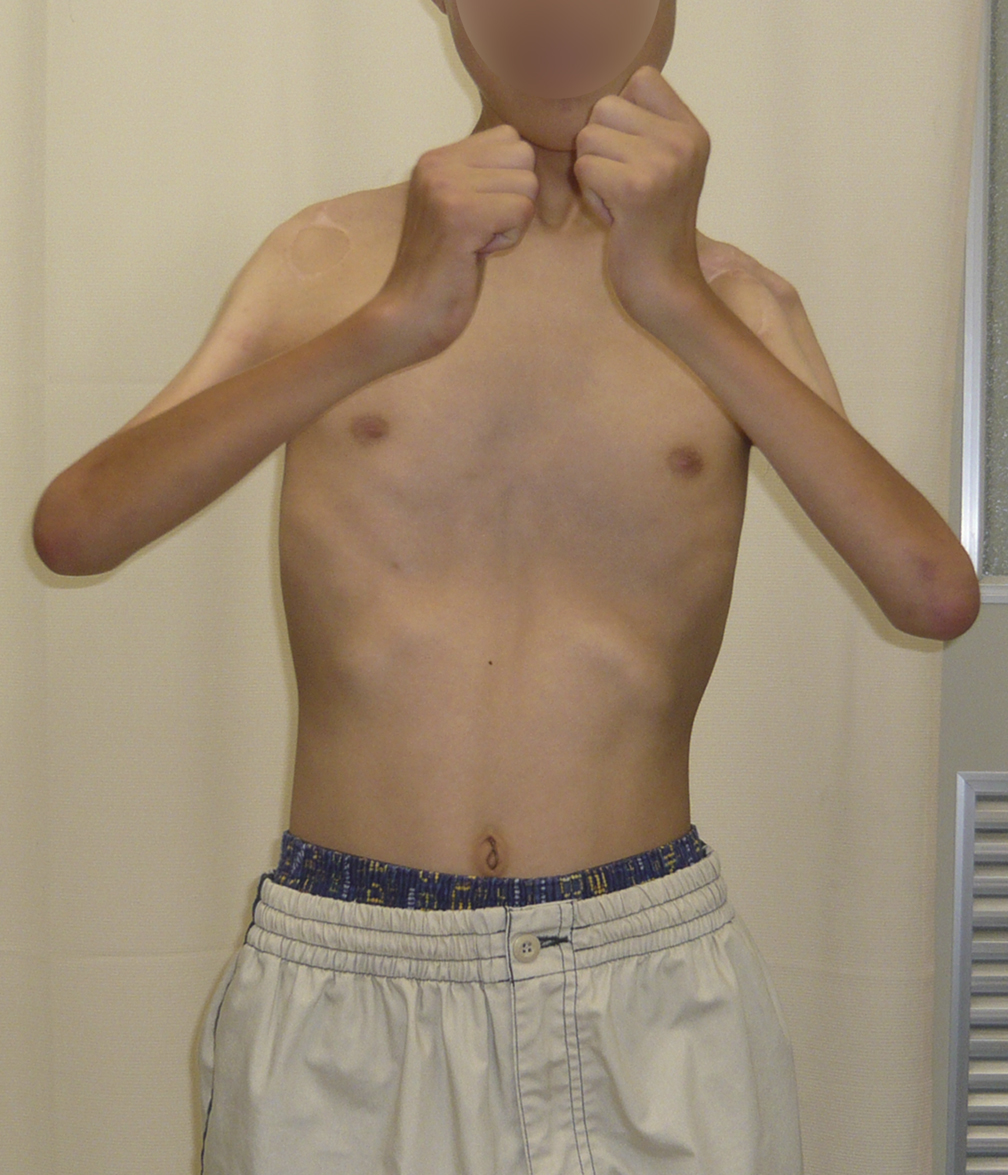
A three-month-old boy with amyoplasia of both upper extremities without lower-extremity involvement was referred to our clinic for treatment (Fig. 1-A). The pectoralis major and latissimus dorsi muscles were hypoplastic and thus could not be used for transfer bilaterally. The patient underwent preoperative physiotherapy to improve passive elbow flexion up to 90° on the right side and 100° on the left side (Table I). Free gracilis muscle transfer was performed for restoration of active flexion at the age of one year and eight months for the right elbow and six months later for the left elbow.
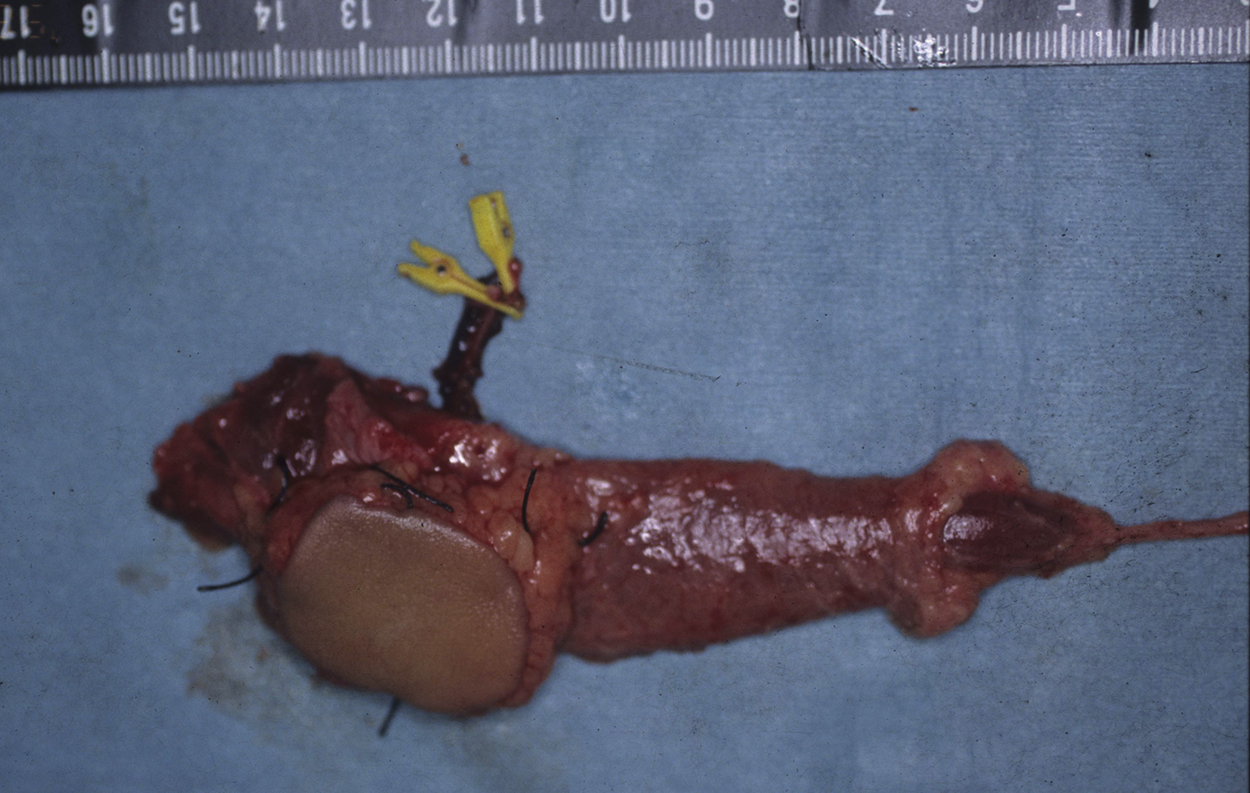
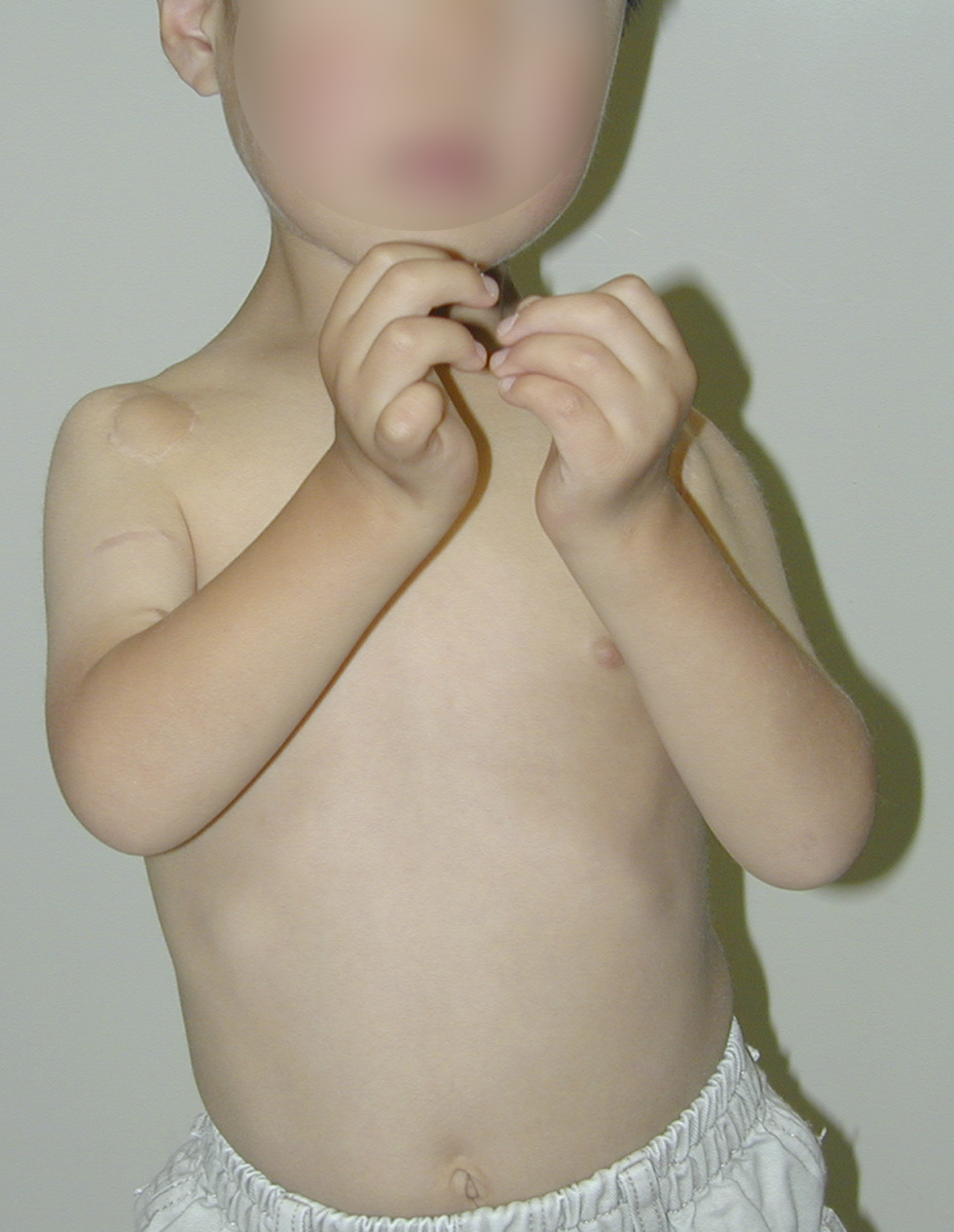
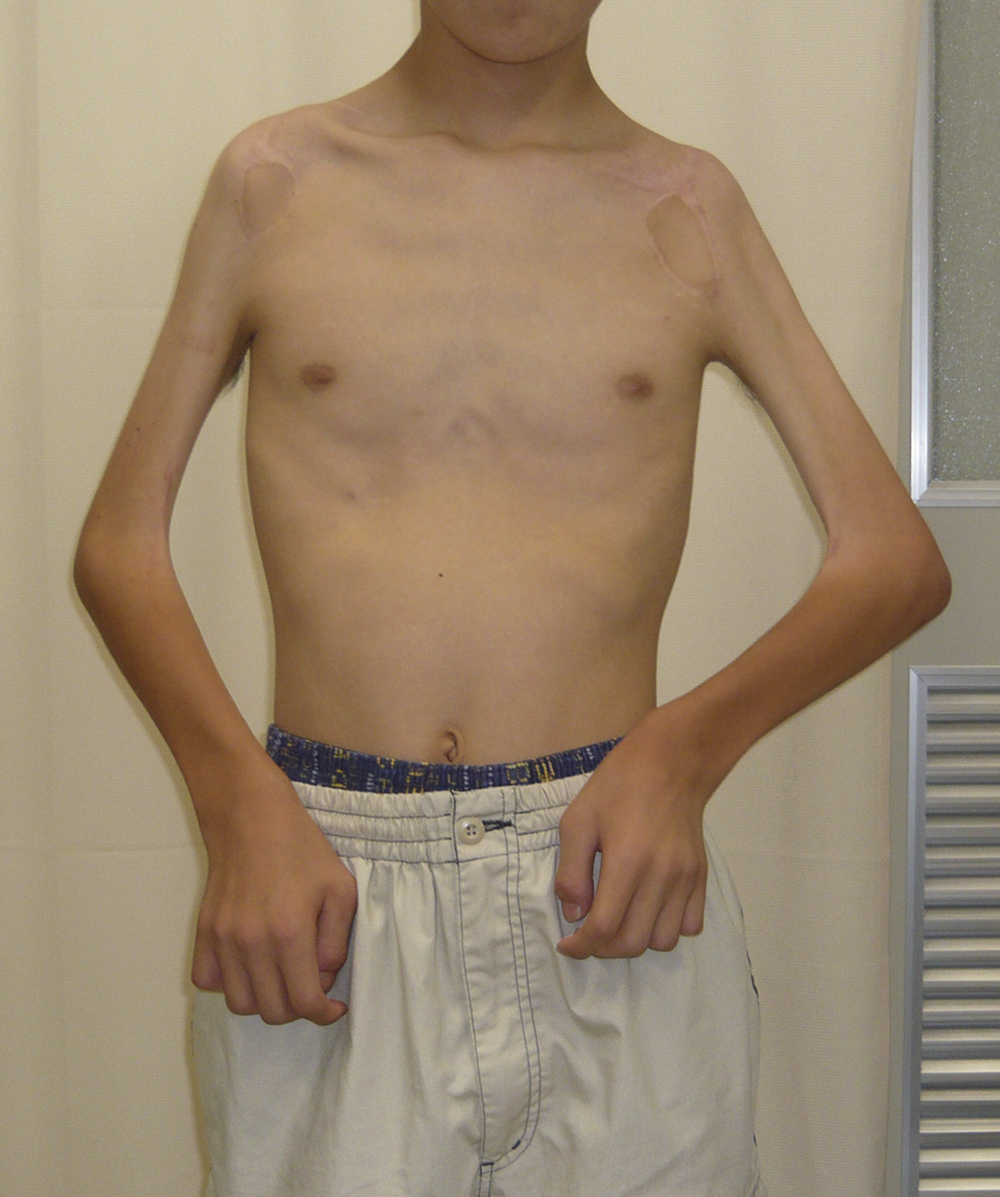
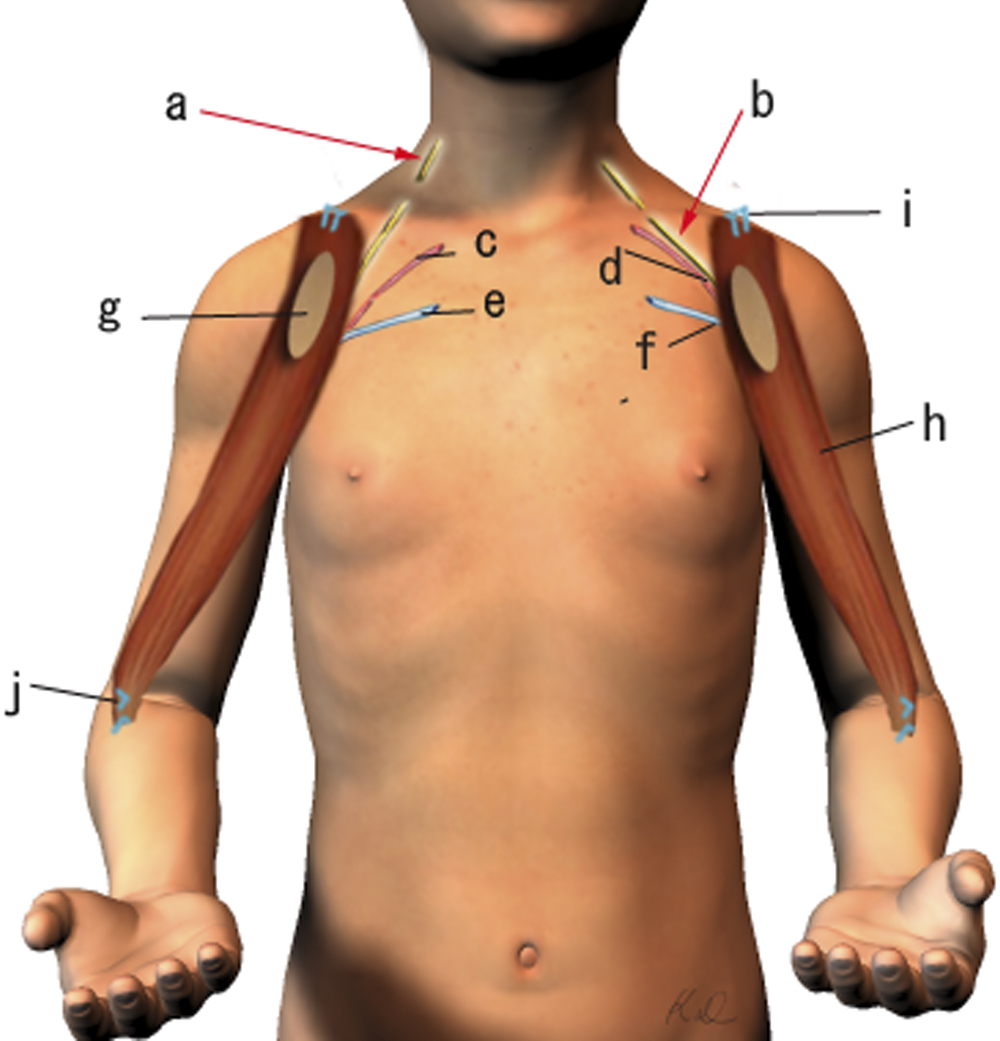
Operative Findings and Procedures The operative findings and procedures were similar on the left and right sides. The biceps brachii, brachialis, and coracobrachialis muscles had a fibrofatty appearance due to degeneration of the muscle fibers. The musculocutaneous nerve was thin, and no contraction was observed in the biceps muscle with electrical stimulation of this nerve. The contralateral gracilis muscle, with an accompanying skin paddle to monitor the vascularity of the muscle graft, was harvested (Fig. 1-B) and was placed in the subcutaneous plane on the anterior part of the deltoid muscle in the upper arm. The distal tendinous portion was passed through the subcutaneous tunnel in the distal part of the arm and fixed to the radial tuberosity through an incision over the cubital fossa (Fig. 2). The motor nerve of the gracilis muscle was passed through the tunnel underneath the clavicle and was sutured to the middle and distal branches of the accessory nerve in the supraclavicular region. Microvascular anastomoses were then performed between the nutrient vessels of the muscle transplant and the thoracoacromial artery and the cephalic vein in the infraclavicular region. The proximal origin of the muscle was fixed to the lateral half of the clavicle with the use of anchoring sutures under maximal traction of the muscle with the shoulder flexed 30° and abducted 30° and the elbow flexed 100°. The muscle flaps, with the accompanying skin paddles, were monitored for vascularity in the early postoperative period. Both of the transferred muscles survived without any postoperative vascular compromise. Reinnervation of the muscles was successfully detected by electromyography three months after surgery, and visible muscle contraction was noted one month later. Electromyographic biofeedback exercises to strengthen the power of the transferred muscles were begun after detection of reinnervation. Postoperatively, active elbow flexion and its power strength increased as time progressed (Figs. 1-C and 1-D). Flexion contracture due to the relative weakness of the triceps brachii developed in the subsequent years; the total arc of active elbow motion was >60°, although the arc shifted to the flexion side. At eleven years postoperatively, the patient had an active range of elbow flexion of 50° to 130° on the right and of 60° to 130° on the left (Figs. 1-E and 1-F). Quantitative analysis of elbow flexion, measured isokinetically with a computerized dynamometer (Kin-Com; Chattanooga Group, Hixson, Tennessee), showed 7 Nm of concentric flexion and 12 Nm of eccentric flexion of the left elbow and 6 Nm of concentric flexion and 8 Nm of eccentric flexion of the right elbow, which is approximately 30% of normal in adults. The patient could perform all of his school and daily living activities without any difficulty or assistance. He was also able to play baseball with his friends.
Proceed to Discussion >>Reference: Doi K, Arakawa Y, Hattori Y, Baliarsing AS. Restoration of elbow flexion with functioning free muscle transfer in arthrogryposis. A report of two cases. J Bone Joint Am. 2011;93:e105. doi:10.2106/JBJS.J.01846
Several operative procedures to improve elbow function of patients with the myopathic type of arthrogryposis multiplex congenita (amyoplasia) have been described. Active elbow flexion can be restored by conventional pedicle muscle transfers with use of the triceps, latissimus dorsi, or pectoralis major muscle, or with the Steindler flexorplasty. However, many of these children do not have donor muscles that are strong enough for transfer. Each of the methods mentioned above has limitations, especially in patients with amyoplasia. Pectoralis major transfer is associated with a decreased effective arc of motion due to flexion deformity developing in the late postoperative period. Deterioration in function could be due to a tenodesis effect as well as a gradual decrease in the amplitude of contraction of the transferred muscle in the presence of a weak triceps muscle. Functional recovery following latissimus dorsi transfer in patients with amyoplasia is less predictable than that in patients with arthrogryposis because of difficulty in assessing muscle mass and strength preoperatively. Similar to the pectoralis major muscle, the latissimus dorsi muscle may be atrophied and infiltrated with fat, leading to suboptimal muscle strength. The long-term results of triceps transfer are worse than those of pectoralis major transfer because of loss of elbow extensor power, which is needed for basic activities like toileting. Another factor to be considered in patients with lower-extremity involvement is that active elbow extension is essential for using a wheelchair, and there may be restriction of motion due to a tenodesis effect. The Steindler procedure provides weak elbow flexion and results in elbow contracture, and preexisting severe wrist palmar flexion and forearm pronation deformities can be exacerbated by proximal relocation of the flexor pronator origin. Functioning free muscle transfer with use of the gracilis muscle is a reliable method for reconstruction after brachial plexus injury, obstetric brachial plexus palsy, and Volkmann contracture, as well as for restoration of function after the excision of tumors. When the lower limb is not involved in patients with amyoplasia, gracilis muscle function is usually normal. When the lower limb is involved, the gracilis muscle may have fat infiltration demonstrated by magnetic resonance imaging (MRI), in which case gracilis muscle transfer is contraindicated. In young children, the free gracilis muscle transfer is a safe technique and reinnervation of the transferred muscle occurs earlier than it does in adults. Delay in reconstruction can result in extension contracture of the elbow, which will require extensive training for effective use of the upper arm. A functioning free muscle transfer in children is technically difficult, but a better outcome can be expected with early intervention. Free muscle transfer in children differs from that in adults because the musculoskeletal structures are smaller. However, the blood vessels and nerves are not proportionately smaller, and the donor and recipient nerves and vessels are usually of adequate size to permit anastomosis without great difficulty. We have observed that the transferred muscle in children with brachial plexus injury and Volkmann contracture grew in proportion to skeletal growth. The gracilis muscle transfer had maintained an arc of elbow motion of >60° at eleven years postoperatively, although the arc gradually shifted to the flexion side because of power discrepancy between the weak triceps muscle and the strong transferred gracilis muscle. This flexion contracture is different from that seen with the pectoralis major transfer, after which the majority of patients develop flexion deformity and a decrease in the effective arc of flexion. It is imperative to perform muscle strengthening and stretching exercises for the transferred gracilis muscle and also for the triceps to avoid secondary flexion contracture following free muscle transfer. The musculocutaneous nerve was not available as a donor motor nerve in our patient, but the intercostal and spinal accessory nerves can be used as donor motor nerves. Spinal accessory nerve dissection is less invasive than intercostal nerve dissection, and the spinal accessory nerve has more motor fibers than the intercostal nerve. Only the terminal branch of the spinal accessory nerve, which innervates the middle and lower portion of the trapezius muscle, is used as a donor motor nerve for the transferred gracilis muscle. The upper portion of the trapezius remains functionally active after the nerve transfer for the gracilis and still acts as a shoulder abductor in patients with amyoplasia involving the upper limb, in whom, because of C5 and C6 nerve root involvement, shoulder function is also affected. Thus, the use of this nerve resulted in a more reinnervated muscle with preservation of the function of the upper fibers of the trapezius and reduced morbidity. The motor nerve of the transferred gracilis was coapted to the spinal accessory nerve in the supraclavicular region without tension by transiently fixing the proximal part of the muscle to the lateral part of the clavicle. Correct muscle tension is critical for good postoperative function. In the literature, most authors have reported that the original muscle length was restored by stretching the muscle until the distance between the markers, which are placed on the surface of the muscle at 5-cm intervals before harvesting, is once again 5 cm. However, we have not used this technique because we harvest the gracilis muscle with the overlying fascia to avoid postoperative adhesion, as the fascia overlying the gracilis muscle does not contract or relax with the gracilis muscle fibers; in addition, the markers of the fascia do not contribute to the landmark for muscle tension. After the distal part of the gracilis muscle was fixed to the radial tuberosity through an incision over the cubital fossa, correct muscle tension was adjusted with the shoulder in 30° of abduction and anterior flexion and the elbow in 100° of flexion. The proximal origin of the muscle was fixed to the lateral half of the clavicle with use of anchoring sutures under maximal traction. Following electromyographic documentation of reinnervation of the transferred muscle (between three and eight months postoperatively), electromyographic feedback techniques with use of small portable myotrainers with surface electrodes were employed to train the transferred muscles to move the elbow and fingers since our patient had difficulty in contracting individual muscles effectively. A prerequisite of this procedure is passive mobility of the upper-extremity joints. Repetitive, gentle, passive manipulation by the therapist and parents is indispensable for achieving passive elbow mobility. It is extremely important and very difficult to provide therapy to an infant before and after surgery. It requires dedicated and skilled therapists as well as active participation by the parents. Preoperatively, >90° of passive elbow flexion should be achieved with physiotherapy or surgical intervention (capsulotomy and triceps lengthening) in order to obtain excellent restoration of elbow flexion with a free gracilis muscle transfer. Compared with conventional procedures for restoration of active elbow flexion in patients with amyoplasia, free muscle transfer achieved optimal function without suitable donor muscles for pedicle transfer. However, this treatment requires a demanding surgical technique and adequately supervised postoperative management.
Reference: Doi K, Arakawa Y, Hattori Y, Baliarsing AS. Restoration of elbow flexion with functioning free muscle transfer in arthrogryposis. A report of two cases. J Bone Joint Am. 2011;93:e105. doi:10.2106/JBJS.J.01846
Spinal accessory nerve, axillary artery, axillary vein
Spinal accessory nerve, thoracoacromial artery, cephalic vein
Suprascapular nerve, suprascapular artery, cephalic vein
Musculocutaneous nerve, anterior circumflex humeral artery, axillary vein


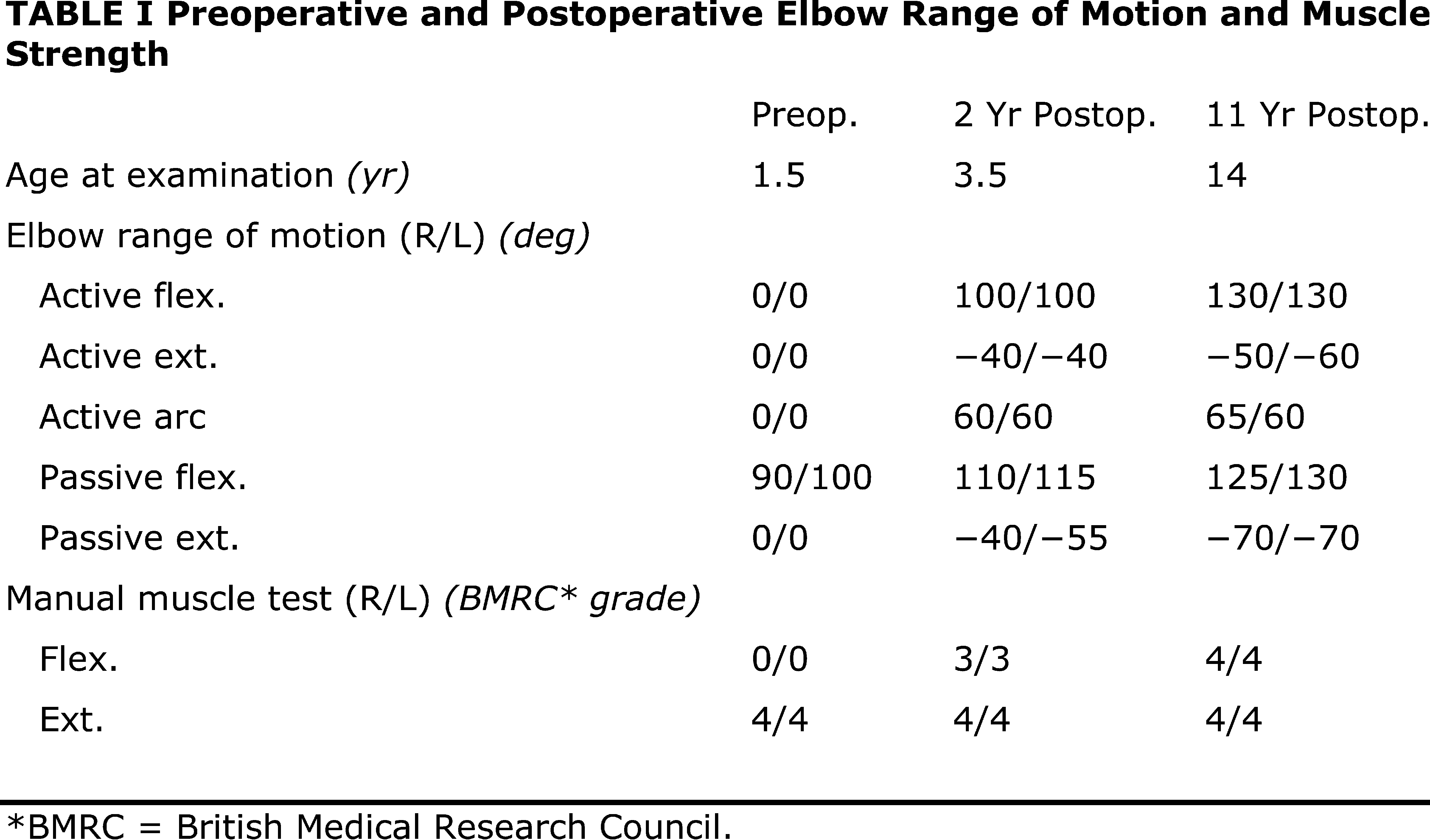
 Fig. 1-A
Fig. 1-A Table I
Table I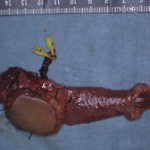 Fig. 1-B
Fig. 1-B Fig. 1-C
Fig. 1-C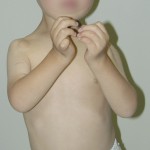 Fig. 1-D
Fig. 1-D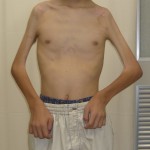 Fig 1-E
Fig 1-E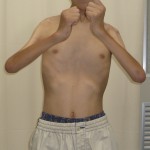 Fig. 1-F
Fig. 1-F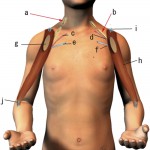 Fig. 2
Fig. 2