A 71-year-old man presented with chief symptoms of left buttock pain and left lower-extremity paresthesias. Of note, he had prior surgical interventions in the lumbar spine and the left lower extremity, including L4-5 microdiscectomy 20 years before presentation and endoscopic left hamstring repair and ischial bursectomy 6 years before presentation. The patient noted the onset of symptoms 1 year after the endoscopic procedure. He localized the pain to the subgluteal region, which was associated with paresthesias radiating down the outer thigh, posterior calf, anterolateral leg, and plantar aspect of the foot. He noted that the symptoms worsened with sitting or sleeping on the involved side. Of note, he described a history of sitting on a hard wallet in the involved region.
The patient had tried multiple interventions for the pain. At 1 year before presentation, he underwent an L3-5 laminectomy at an outside hospital for a presumed diagnosis of spinal stenosis. He had also undergone bilateral sacroiliac joint injections and ipsilateral piriformis injection the year before presentation. The patient took a regimen of duloxetine, oxycodone, and tramadol to help mitigate the pain. None of these medications or treatments provided the patient with bearable symptomatic relief.
On physical examination, the patient was found to be tender to palpation in the left deep gluteal region. He had reproducible pain with hip abduction and external rotation (Pace’s sign) and hip flexion, adduction, and internal rotation (FAIR) test. He did not have any pain with hip extension and internal rotation (Freiberg’s sign). He demonstrated full strength in all muscle groups and was sensate in all distributions distally.
The initial differential diagnoses included lumbar radiculopathy and piriformis syndrome. Magnetic resonance imaging (MRI) scans of the lumbar spine and pelvis were acquired and were interpreted as unremarkable. No nerve root compression in the lumbar spine or sciatic nerve compression by the piriformis tendon was noted. Hoffmann reflexes on a nerve conduction study (NCS) were found to be delayed in the FAIR position compared with neutral positioning in the tibial nerve distribution.
Although the patient’s clinical examination and electrodiagnostic studies supported a working diagnosis of piriformis and deep gluteal syndrome, a definitive diagnosis could not be reached because of the negative imaging findings. Options ranging from continued nonoperative treatment to surgical exploration and decompression were discussed with the patient. After a thorough discussion of the risks and benefits, he elected to proceed with surgical intervention.
The surgical procedure was performed using a Kocher-Langenbeck approach with the patient in the prone position. After incision of the gluteus fascia, the gluteus maximus fibers were released proximally and the iliotibial tract was released distally. This revealed the trochanteric bursa, which appeared fibrous and expansile in nature. Surprisingly, this abnormal tissue extended deep to encapsulate the short external rotators and the sciatic nerve distally. This abnormal tissue was dissected free, and the sciatic nerve was followed proximally, revealing the piriformis muscle belly and insertion (Fig. 1). The sciatic nerve was found to be compressed at the piriformis insertion site. After careful visualization of the nerve, a piriformis tenotomy was performed and the entire muscle was resected. The abnormal fibrous tissue was sent for pathology.
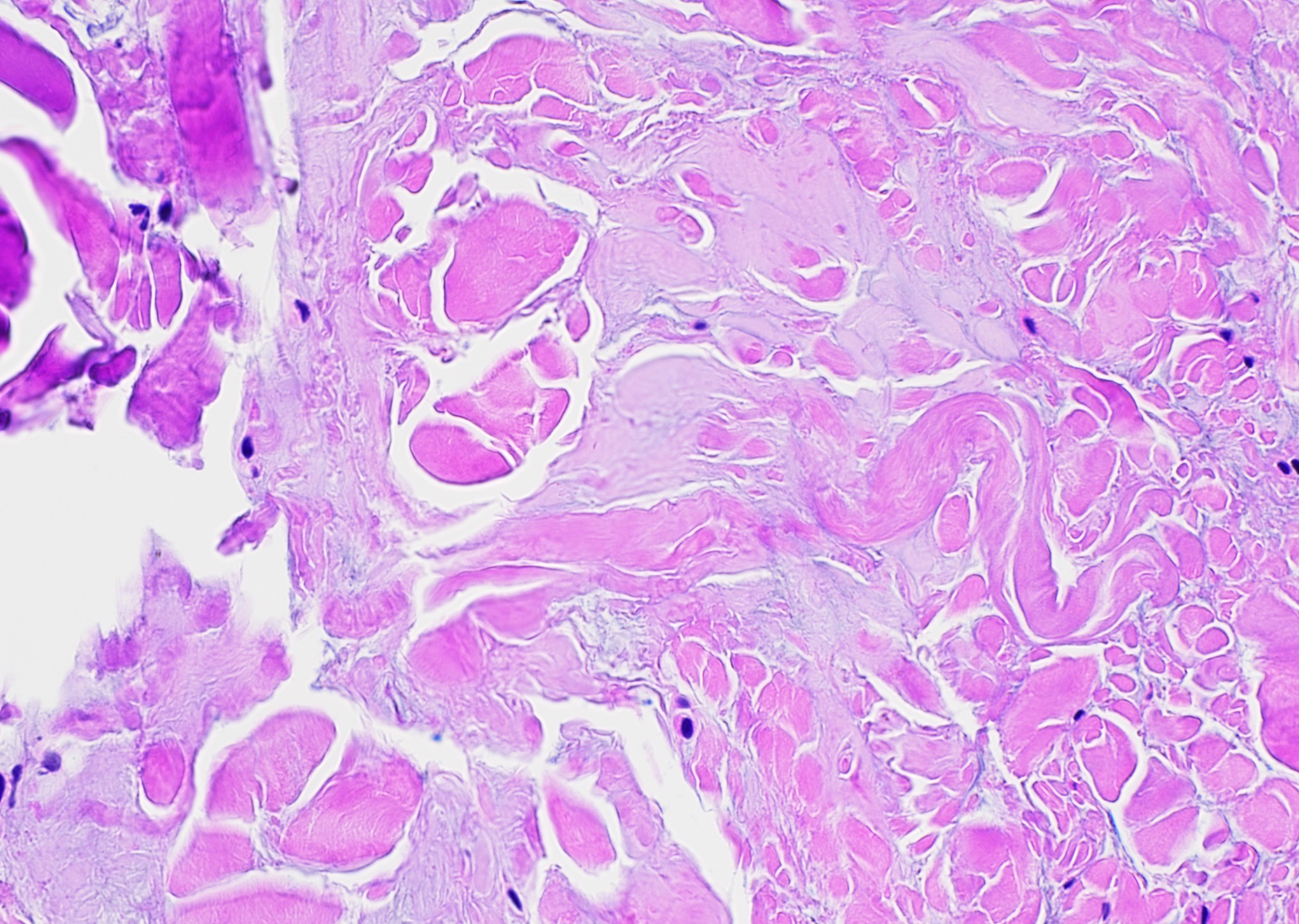
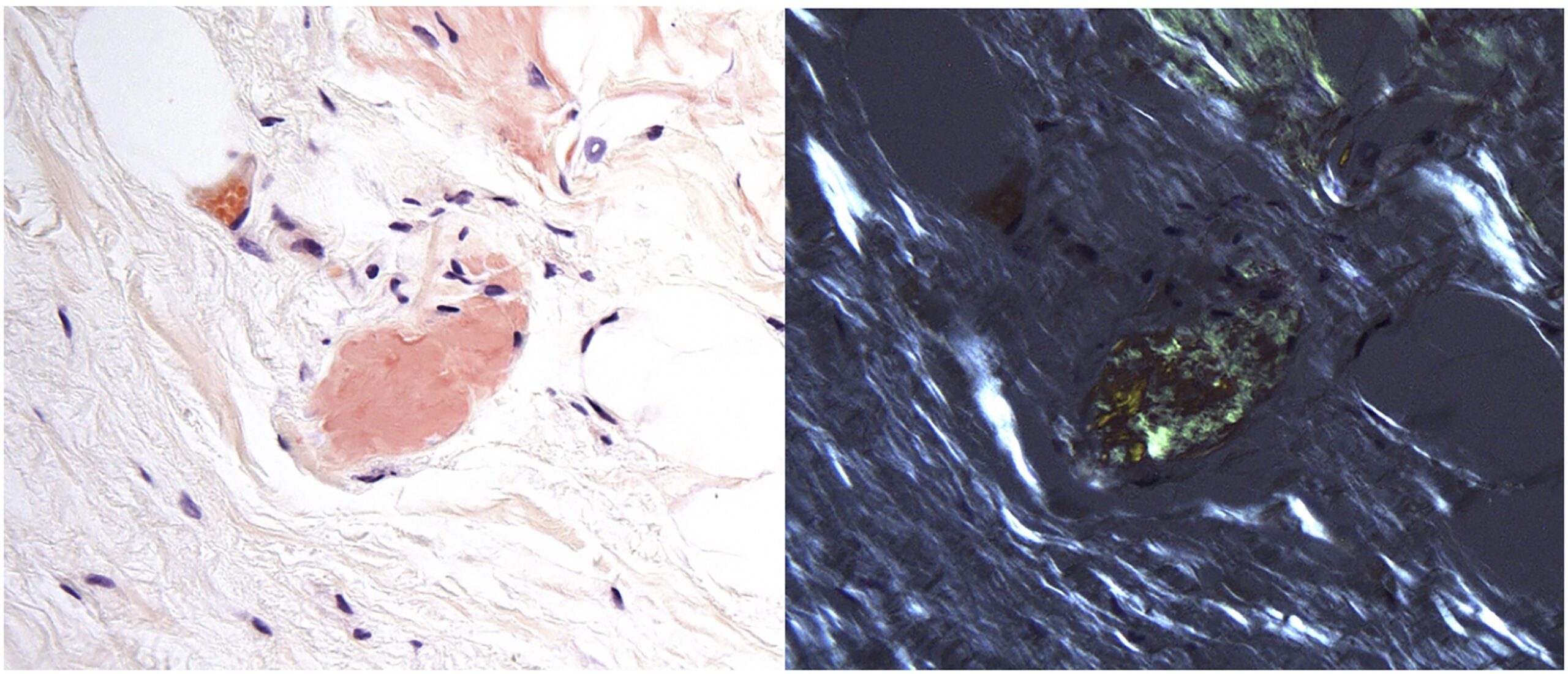
Pathology revealed pale-stained fibrillar material that stained positive with Congo red and showed apple-green birefringence when viewed with polarized light, indicating amyloid deposition (Figs. 2 and 3), and the patient was diagnosed with amyloidoma. The amyloid was subsequently identified more specifically by liquid chromatography as transthyretin protein.
Postoperatively, the patient was treated with a multimodal pain medication regimen (including neuropathic medications). He was allowed to bear weight as tolerated without restriction. He was able to ambulate without difficulty and was ultimately discharged from the hospital on postoperative day 2.
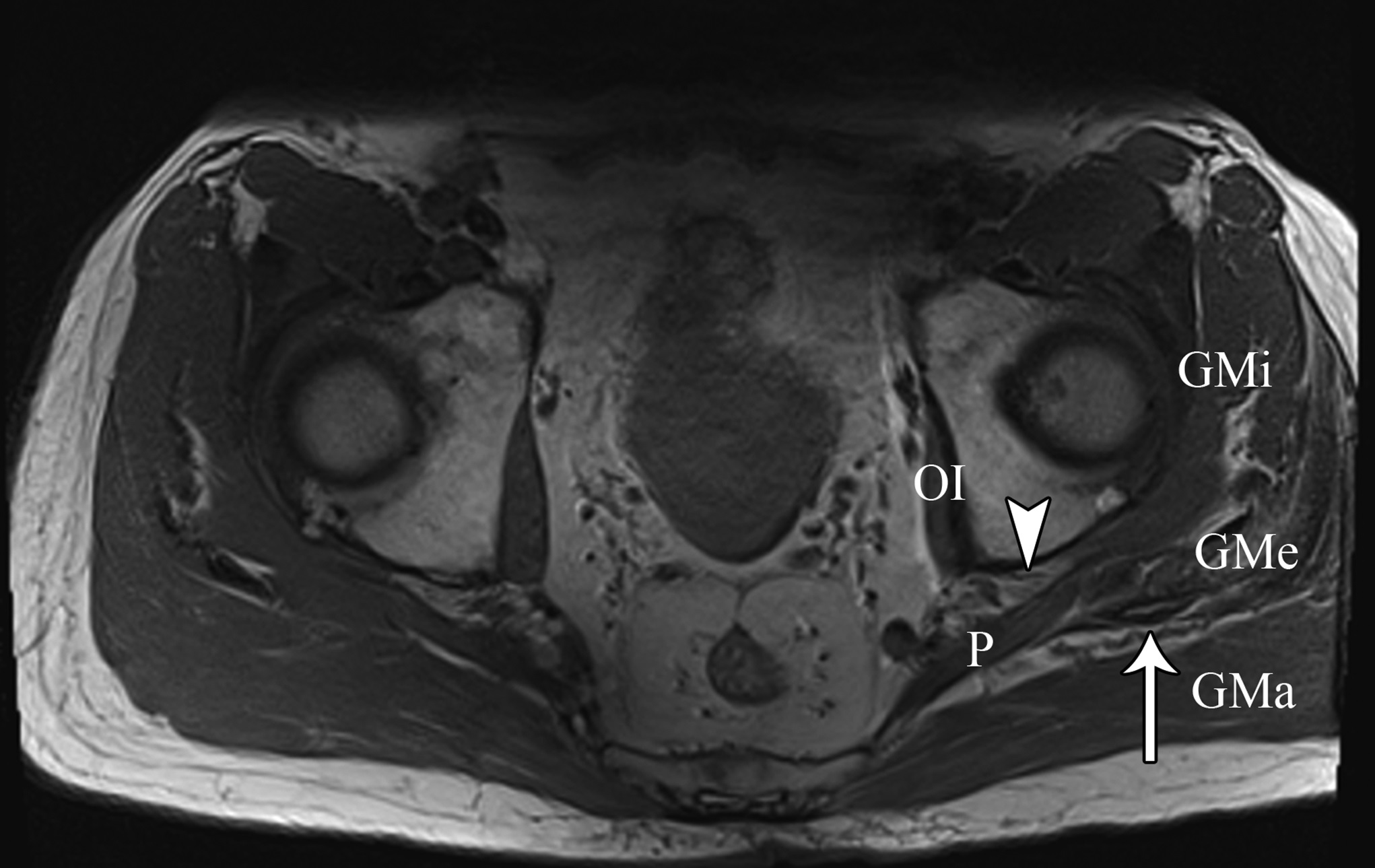
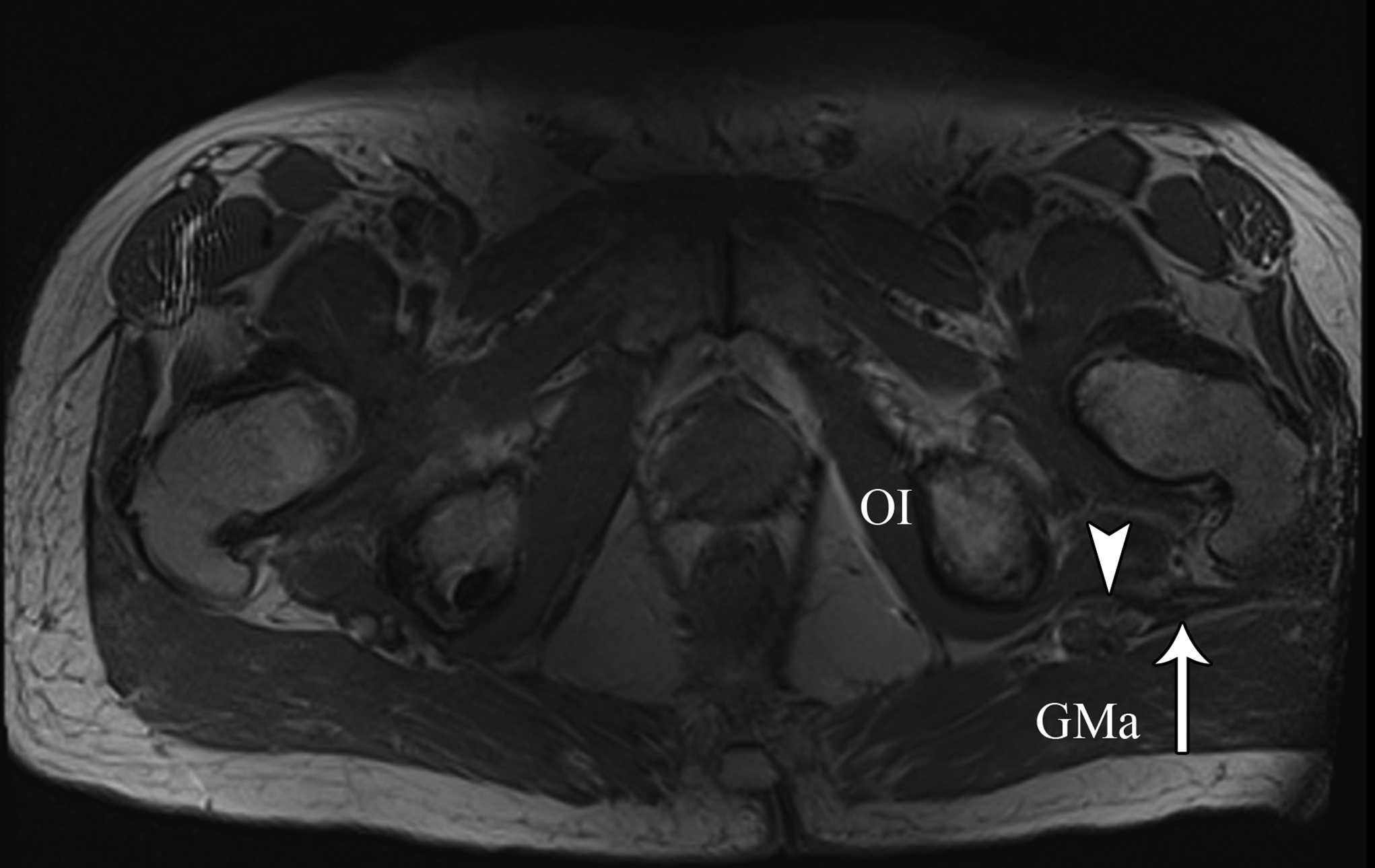
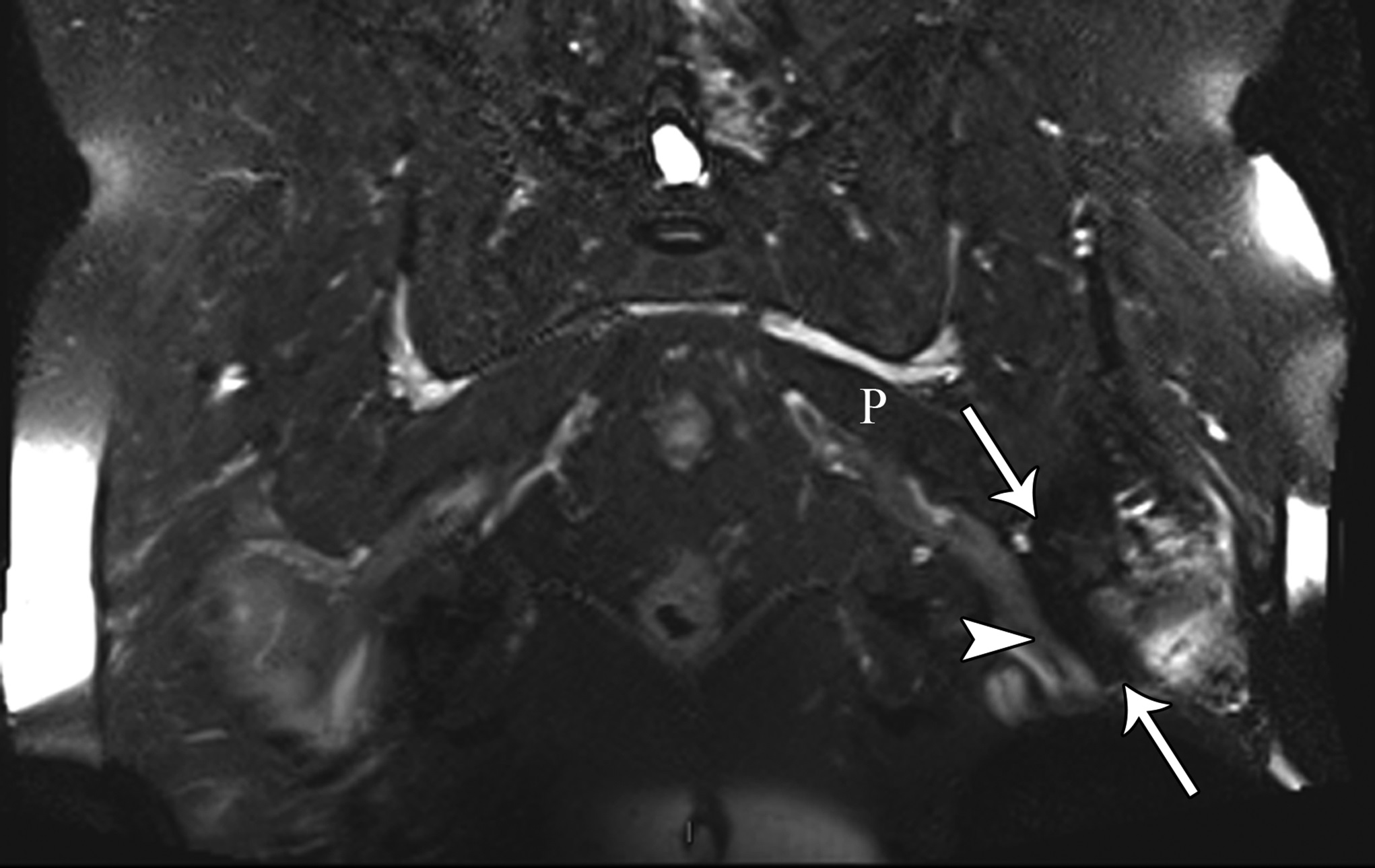
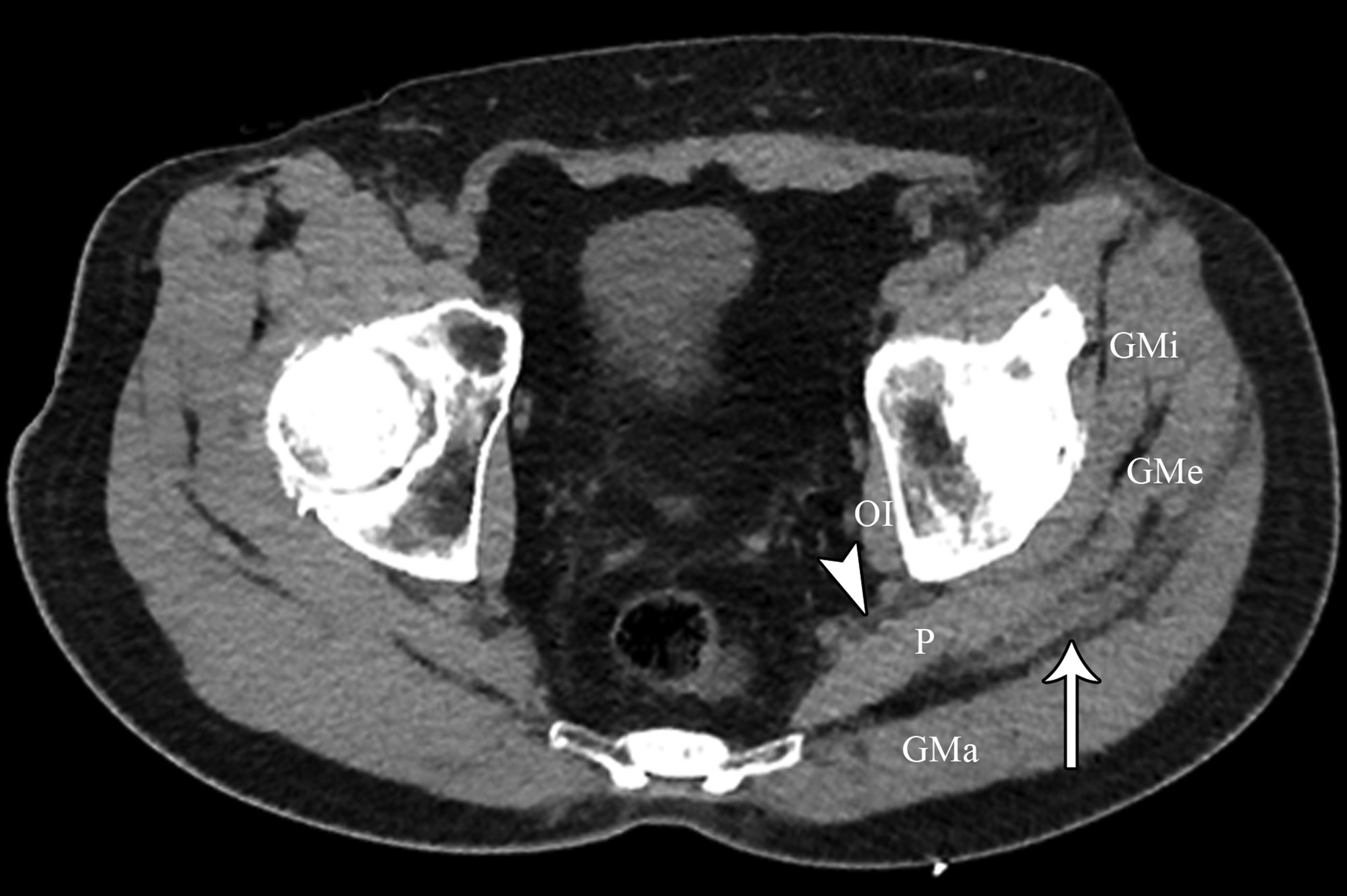
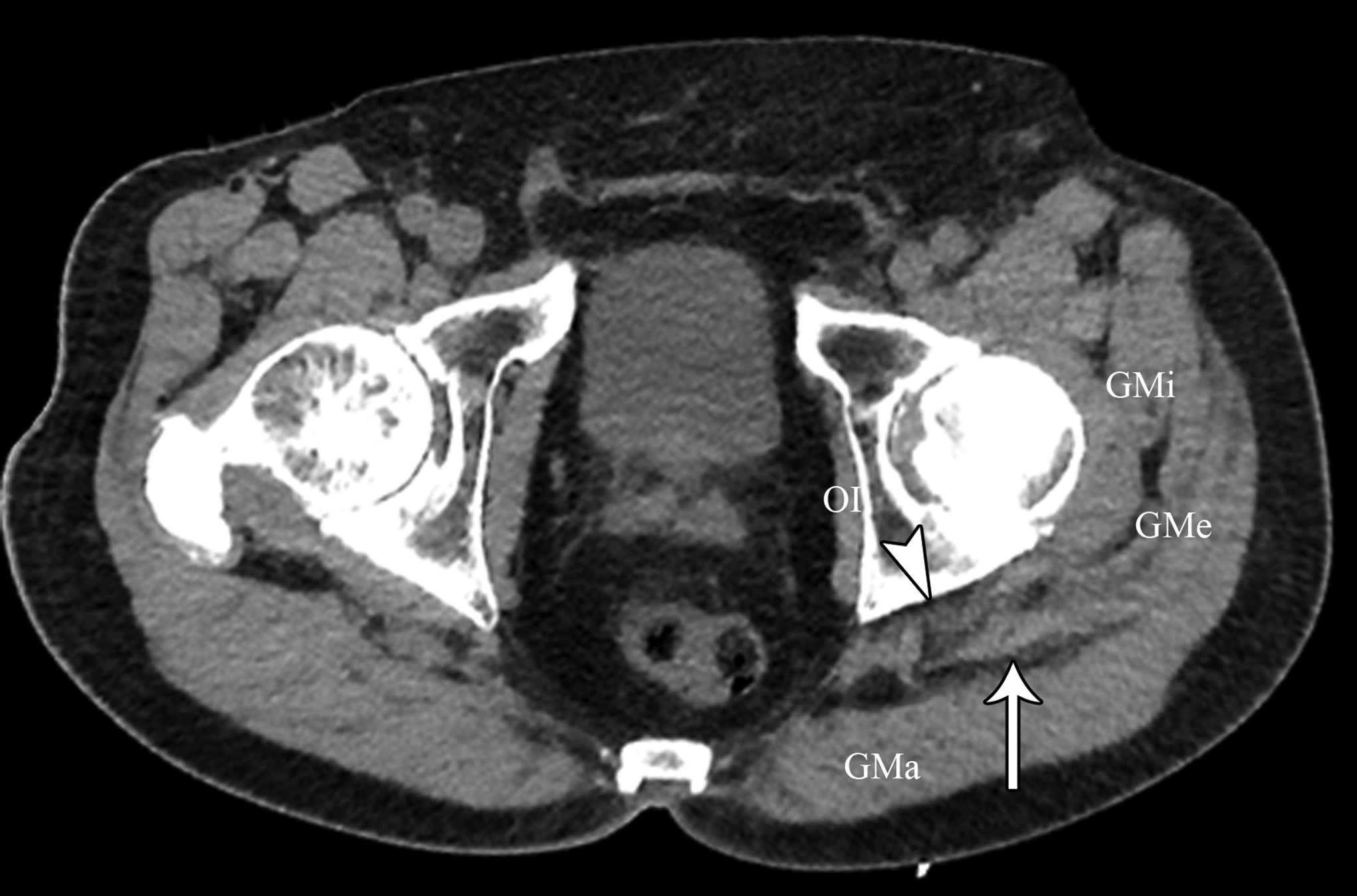
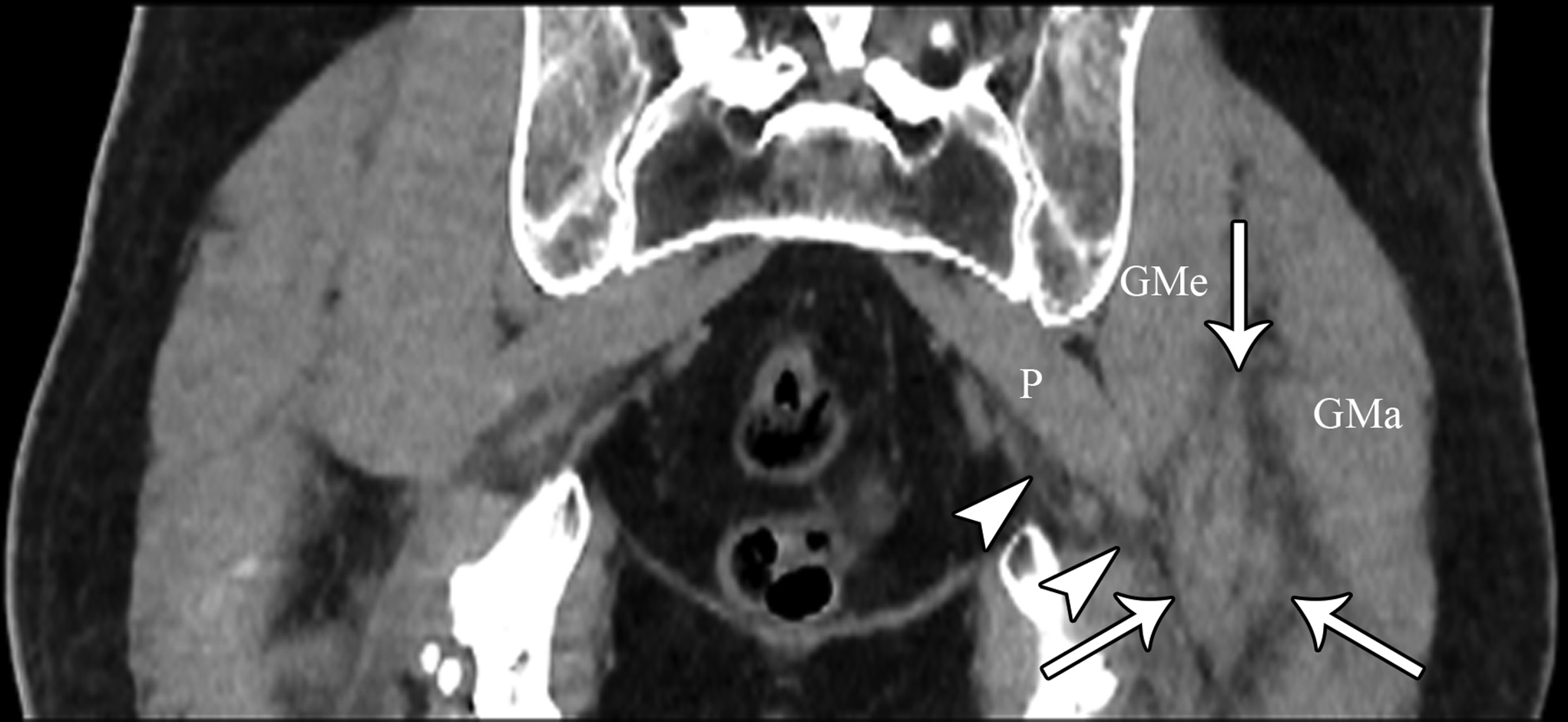
At the 2-week postoperative follow-up, the patient demonstrated excellent recovery with substantial improvement in the initial pain and paresthesias, and he had continued to have improved symptoms at 1 year postoperatively. The MRI scan was retrospectively reviewed given the intraoperative findings, and a hypointense lesion on T1-weighted imaging was noted in the intramuscular plane between the gluteus medius and the gluteus minimus (Figs. 4-A, 4-B, and 4-C). The soft-tissue mass was also present on computed tomographic (CT) imaging with near muscle density (Figs. 5-A, 5-B, and 5-C).
Proceed to Discussion >>Reference: : Parikh HB, Matcuk GR, Leong M, Vrahas MS, Seruya M. Amyloid deposition: an unusual case of deep gluteal syndrome and sciatic nerve compression: a case report. JBJS Case Connector. 2022 July-September;12(3):e22.00075.
Patients with extremity pain and paresthesias pose a diagnostic challenge. Both spinal and extraspinal causes must be considered, including piriformis and deep gluteal syndrome. A proposed etiology includes blunt trauma to the buttock. This is believed to result in hematoma formation that leads to a compressive neuropathy of the sciatic nerve from subsequent scar formation. We initially suspected that the patient’s symptoms were secondary to trauma from sitting on a hard wallet. However, the surprise intraoperative finding of fibrous tissue led to a diagnosis of soft-tissue amyloidoma on pathological evaluation, a solitary mass of amyloid. Of note, the MRI findings seen in our patient were consistent with prior reports of soft-tissue amyloidoma, demonstrating hypointense enhancement on T1 and T2-weighted sequences without invasion into the bone.
This diagnosis is exceedingly rare, with amyloidomas mainly found in the groin or the abdominal wall. Amyloidosis results from the extracellular deposition of insoluble amyloid proteins, ultimately resulting in organ dysfunction. Causes of amyloidosis include genetic, autoimmune, and acquired disorders and may be secondary to hemodialysis, multiple myeloma, or drug injections. Amyloid can be deposited throughout the body, including in the oral mucosa, nail undersurfaces, heart, respiratory tract, and kidneys. The gold standard for diagnosis is tissue biopsy, notably using Congo red staining that appears pale green when viewed with polarized light. Treatment options include chemotherapy, organ transplantation, and other supportive measurements catered to symptomatic relief. Our patient did not demonstrate an underlying autoimmune disorder or a plasma cell dyscrasia on further workup, leading to low suspicion of AA or AL amyloidosis.
To the best of our knowledge, this is the first report of amyloidosis as an etiology of piriformis and deep gluteal syndrome and sciatic nerve compression. Of note, this disease process has been implicated in other entrapment neuropathies, including carpal tunnel syndrome. A previous report showed an incidence of 34% of amyloid deposition in patients with idiopathic carpal tunnel syndrome. In addition, TTR deposition in the flexor tenosynovium occurs approximately 5 to 10 years before the development of cardiac amyloidosis. Thus, patients found to have carpal tunnel syndrome with TTR deposition should undergo close follow-up with appropriate cardiac imaging. Our patient did not demonstrate symptoms of carpal tunnel syndrome, as he had negative Phalen’s and Durkan’s compression tests.
Prior reports have identified amyloidosis within the hip in patients undergoing hemodialysis. In addition, deposits have been reported to be found within bursal spaces, including subacromial, olecranon, iliopectineal, and popliteal bursae, believed to be due to the accumulation of β2-microglobulin.
Although bursal involvement was found intraoperatively, the extent of fibrosis (ultimately amyloid deposition) was well beyond the bursal space into the musculature of the posterior compartment of the thigh. Interestingly, our patient did not have a history of kidney disease necessitating hemodialysis. As such, further workup is warranted.
In this report, we present a case of a patient who was initially presumed to have atypical piriformis and deep gluteal syndrome and was later diagnosed with an amyloidoma. To the best of our knowledge, this is the first report of amyloid deposition as the etiology for piriformis and deep gluteal syndrome and sciatic nerve compression. This diagnosis has prompted further workup of systemic amyloidosis. Of note, preoperative electrocardiogram and postoperative echocardiography were performed and were found to be unremarkable. However, our understanding of amyloidosis and peripheral nerve syndromes remains rather limited. When investigating potential causes, providers should remain cognizant of amyloidosis and soft-tissue amyloidoma as a potential diagnosis. This index of suspicion may be higher in patients who do not present with classic clinical examination and electromyogram and NCS findings, as seen in this patient.
Reference: : Parikh HB, Matcuk GR, Leong M, Vrahas MS, Seruya M. Amyloid deposition: an unusual case of deep gluteal syndrome and sciatic nerve compression: a case report. JBJS Case Connector. 2022 July-September;12(3):e22.00075.
What is the diagnosis?
Retroperitoneal fibromatosis
Ischemic myonecrosis
Schwannoma
Amyloidoma
Gossypiboma

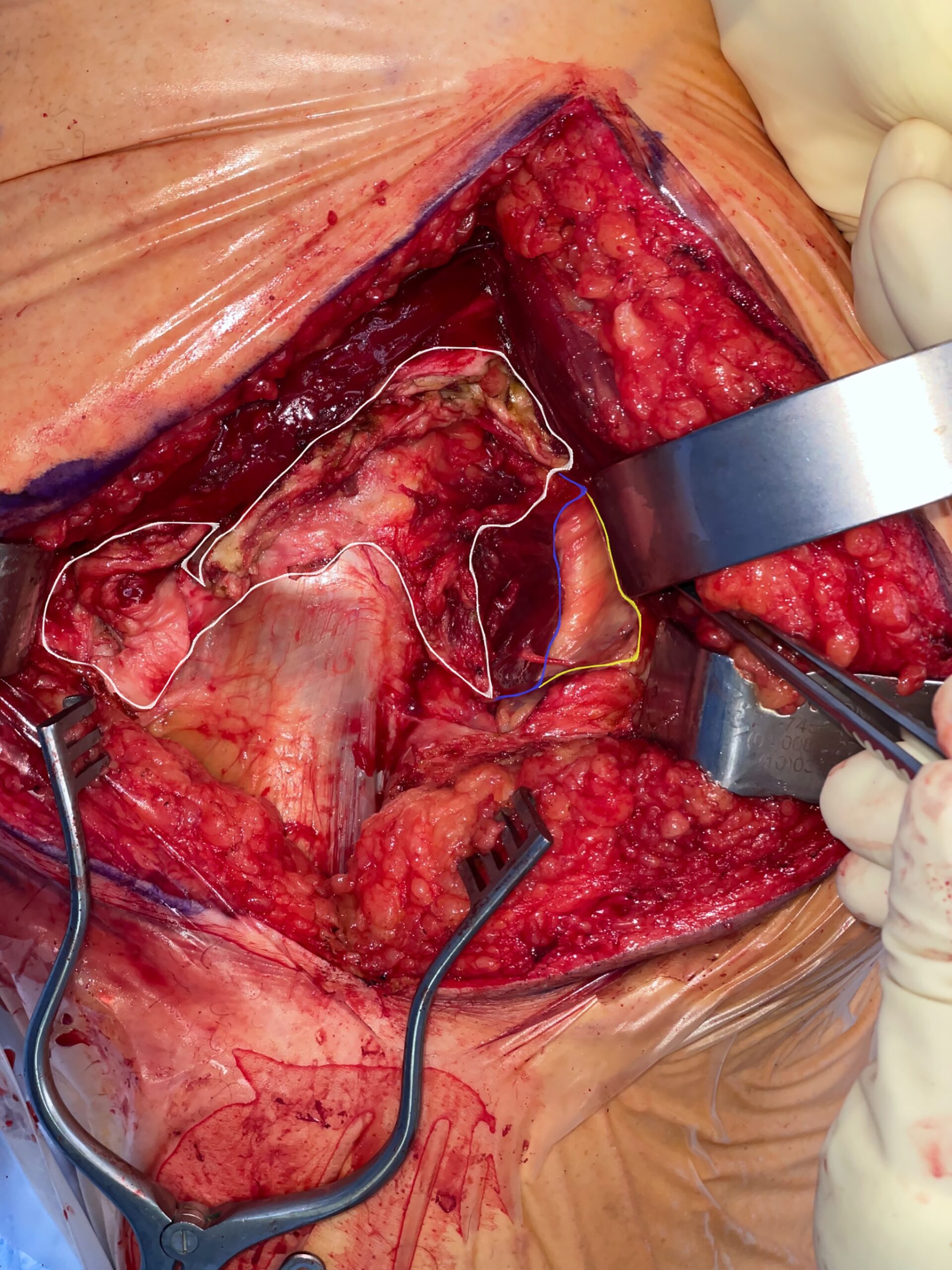
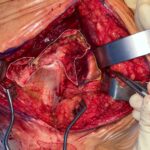 Fig. 1
Fig. 1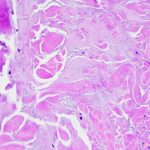 Fig. 2
Fig. 2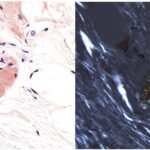 Fig. 3
Fig. 3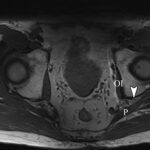 Fig. 4-A
Fig. 4-A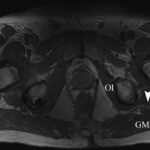 Fig. 4-B
Fig. 4-B Fig. 4-C
Fig. 4-C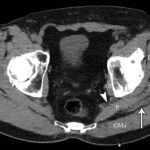 Fig. 5-A
Fig. 5-A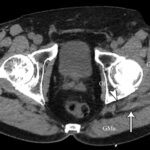 Fig. 5-B
Fig. 5-B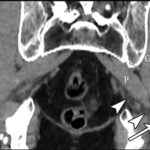 Fig. 5-C
Fig. 5-C