A Sixty-eight-Year-Old Woman with Shoulder and Knee Pain
February 1, 2012
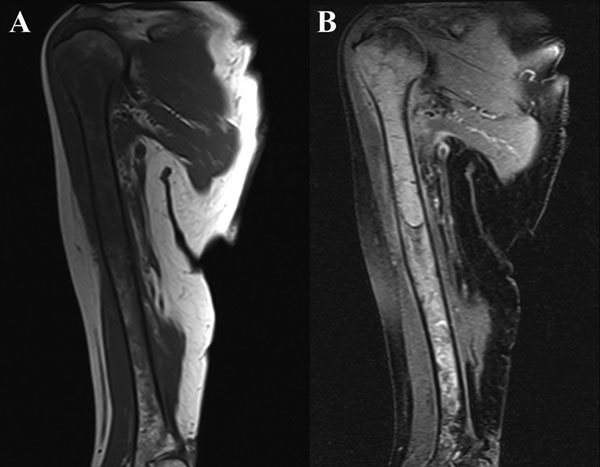
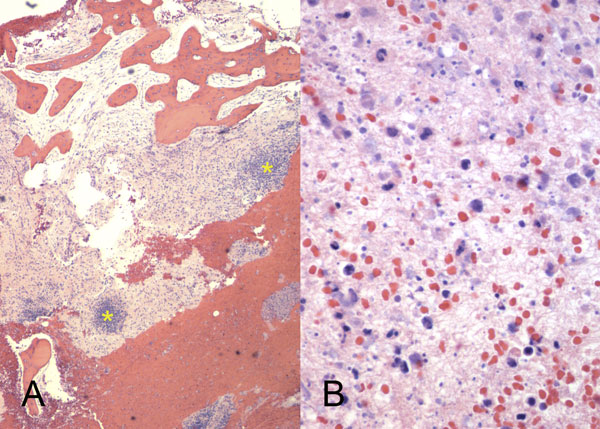
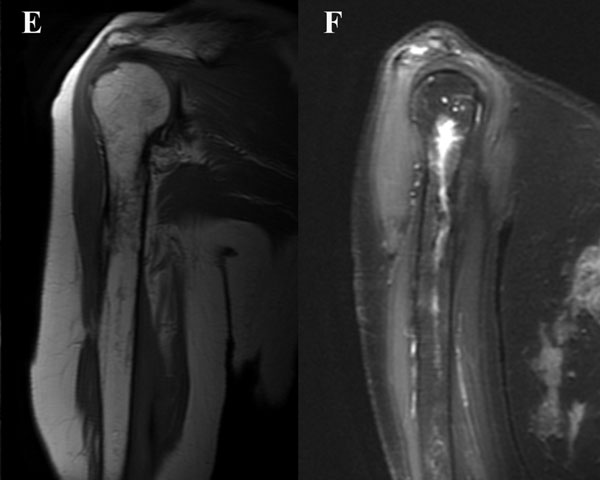
A sixty-eight-year-old woman presented to our orthopaedic office with severe pain in the right shoulder and mild pain in the right knee. She had been diagnosed as having Waldenström macroglobulinemia seventeen years earlier during a workup for osteoporosis. She was treated with chemotherapy (rituximab), and the disease remained well controlled. Her medical history was notable only for mild mitral valve prolapse, for which she usually took oral antibiotics before dental procedures. She had not, however, taken antibiotics prior to her most recent dental cleaning six months prior to presentation. Pain developed in the right shoulder and knee three months prior to presentation at our institution. She had also lost 10 lb (4.5 kg) and had occasional night sweats, but reported that she had had no fevers. Anti-inflammatory medications partially relieved the pain. During the subsequent months, the shoulder pain worsened. On physical examination, the patient demonstrated full motion of the shoulder with a dull ache throughout the arc of motion. She had mild tenderness to palpation of the soft tissues around the shoulder with palpable soft-tissue fullness, but there was no localized swelling or erythema. She demonstrated full motor strength in all muscle groups and had no sensory deficits. All distal pulses were palpable. The examination of the knee had unremarkable findings, including a full, painless range of motion. There was no effusion or joint line tenderness, and examination of the cruciate and collateral ligaments had normal findings as well. She exhibited a normal gait. The laboratory data showed inflammatory markers within normal limits; recent immunoelectrophoresis revealed monoclonal IgM antibodies with low levels of gamma globulin (0.4%; normal, 0.6% to 1.9%), IgG (210 mg/dL; normal, 639 to 1349 mg/dL), and IgA (<6.67 mg/dL; normal, 70 to 312 mg/dL), which resonance imaging (MRI) examination of the right shoulder revealed extensive intramedullary and mild extramedullary edema, with enhancement in the bone and periosteum extending into the soft tissue around the proximal part of the humerus (Fig. 1-A and Fig. 1-B). She was admitted to the hospital for an open biopsy of the proximal part of the humerus and surrounding soft tissue. Operative exploration revealed 300 mL of purulent material in the subacromial and subdeltoid spaces as well as in the axillary pouch. Inspection of the proximal part of the humerus demonstrated intact cortices without any signs of draining sinuses or tracts connecting into the intramedullary canal. Because the patient had persistent knee pain, an MRI of the right thigh was acquired and revealed intramedullary signal changes with periostitis and soft-tissue enhancement. Biopsies of both the humerus and the distal femur were obtained (Figs. 2-A and 2-B, Figs. 3-A and 3-B).
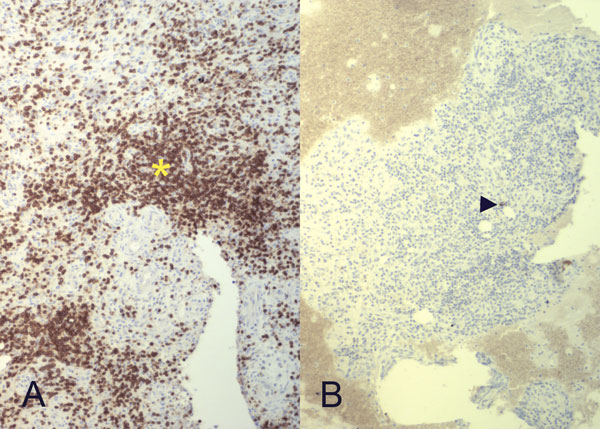
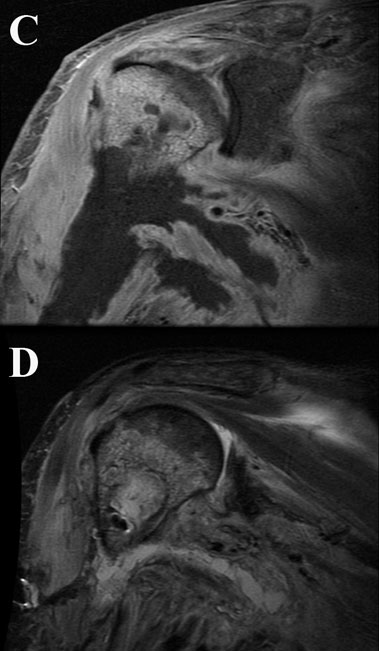
Intraoperative cultures grew pansensitive Streptococcus pneumoniae. A peripherally inserted central catheter was placed and intravenous ceftriaxone administered. Repeat MRI of the shoulder three days after surgery revealed extensive intramedullary edema and enhancement, consistent with osteomyelitis, with a large intramedullary abscess measuring 2.0 × 1.5 × 7.3 cm in craniocaudal dimension (Fig. 4). A sinus tract extended from the intramedullary abscess through the anteromedial cortex of the proximal part of the humerus into the axilla, where there was another large peripherally enhancing fluid collection measuring 5.8 × 3.7 × 5.5 cm (Fig. 4). Given these abnormal MRI findings, the patient was returned to the operating room for open debridement of the humeral shaft and open biopsy of the lesion in the distal end of the right femur. Tissue specimens were sent for histologic examination, and both showed areas of necrosis and abscess formation with a dense lymphoid infiltrate, consistent with abscess and acute-on-chronic osteomyelitis (Fig. 2). Both locations revealed proliferation and aggregation of T cells without an increase in B-cell concentration, indicating a reactive lymphocytosis without any evidence of B-cell non-Hodgkin lymphoma or other malignant transformation (Fig. 3). The intraoperative cultures from both sites, however, were negative, likely because of the prior antibiotic therapy. Postoperatively, a Sarmiento cast brace was applied to the shoulder and a knee immobilizer was used to protect the knee from secondary fracture during postoperative rehabilitation. A transesophageal echocardiogram revealed mitral valve prolapse with no evidence of vegetation. Further investigations to reveal a source of infection were negative. The patient was treated with a six-week course of intravenous ceftriaxone. At the time of the one-year follow-up, she had resumed her functional daily activities without any pain. The physical examination of the shoulder and knee revealed full range of motion without any signs of infection or inflammation. The C-reactive protein level was <0.5 mg/dL, the most recent white blood-cell count was 4800 cells/mm3, and two sets of blood cultures were negative. The MRI acquired at the one-year postoperative follow-up evaluation showed T1-weighted hypointense and STIR hyperintense signal abnormality in the proximal part of the humerus, which is nonspecific and may represent granulation tissue; marked interval decrease in soft-tissue edema is consistent with resolution of myositis and cellulitis. There was no evidence of active infection (Fig. 5).
Proceed to Discussion >>Reference: Fabricant PD, Unnanuntana A, Hartman BJ, Lane JM. Multifocal osteomyelitis with Streptococcus pneumoniae in a patient with Waldenström macroglobulinemia. A case report. J Bone Joint Surg Am. 2011;93:e46(1-5).
Waldenström macroglobulinemia is a rare blood dyscrasia. Similar to multiple myeloma, it is a malignant disease of B lymphocytic cells; however, it is distinct from multiple myeloma in that cystic bone lesions are rare and patients present with lymphadenopathy, hepatosplenomegaly, and hyperviscosity syndrome. Because of the similarity with B-cell lymphomas, patients with Waldenström macroglobulinemia who manifest skeletal symptoms raise concern for malignant transformation to B-cell lymphoma such as higher-grade non-Hodgkin lymphoma. Musculoskeletal manifestations of Waldenström macroglobulinemia, including septic arthritis and one case of lumbar vertebral body osteomyelitis in conjunction with disseminated Nocardia infection, are rare but have been reported. Lastly, the diagnosis of infection is difficult because these patients often have abnormal MRI findings from marrow involvement of the underlying disease. Paramount to understanding the implications of vulnerability to infection in this patient is knowledge of the immune process that clears pyogenic encapsulated infections. In this patient, the infection was able to flourish because of factors specific both to the organism as well as to the patient’s decreased systemic immunity. Studies have shown that, similar to patients with multiple myeloma who produce high levels of monoclonal IgG or IgA, patients with Waldenström macroglobulinemia have an increased level of monoclonal IgM antibodies that leads to reduced levels of normal immunoglobulin by reduced synthesis and/or increased catabolism. In addition, patients with Waldenström macroglobulinemia have an impaired primary and secondary antibody response to antigen administration and immunizations. This abnormality renders patients more vulnerable to pyogenic infections, particularly with encapsulated organisms such as Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitides. Furthermore, chemotherapy for these diseases with alkylating agents, steroids, and newer nucleoside analogs, such as fludarabine and cladribine, can cause substantial myelosuppression and reduced systemic T-cell immunity and can increase the risks for both routine and opportunistic infections. Although Waldenström macroglobulinemia is exceedingly rare, this concept is applicable to several disease states. As mentioned above, multiple myeloma (as well as plasma cell disorders as a whole) produces analogous pathophysiology, as such disorders also create monoclonal antibodies and immunity is decreased by the same mechanism. In addition to rare hematologic malignant diseases, asplenia confers the same vulnerability. Because the spleen is the final common step in the course of clearing encapsulated bacteria opsonized with normal antibody, these patients are "essentially asplenic" in this regard. Unfortunately, unlike patients with traumatic or surgical splenectomy or autosplenectomy, patients with hematologic malignant diseases such as Waldenström macroglobulinemia and multiple myeloma do not produce effective antibodies in response to immunization to encapsulated organisms. This difference makes them especially susceptible to infection with these bacteria. While patients with immunoglobulin deficiencies cannot opsonize encapsulated organisms for presentation to the spleen for clearance, anatomically or functionally asplenic patients produce adequate antibodies, but the opsonized bacteria are not cleared by the (nonfunctioning or absent) spleen and must be cleared by other means. Both, however, lack the natural ability to fight infection with encapsulated organisms. Additionally, more common disorders that render a patient essentially asplenic with regard to bacterial susceptibility include any state in which the physiology of opsonization and clearance of encapsulated organisms is hindered. These include acquired asplenia due to splenic rupture from trauma or tumor, surgical splenectomy or embolization (e.g., to treat idiopathic thrombocytopenic purpura, thalassemia, or spherocytosis), or autosplenectomy (underlying diseases that destroy the spleen, such as sickle-cell disease). Patients living in any of these asplenic states are known to be at risk for infection with encapsulated organisms, and infections can manifest in any organ system, with the most severe infection being overwhelming postsplenectomy infection. Despite its rarity in 0.5% of splenectomized patients, the consequences of overwhelming postsplenectomy infection are dire, as the mortality approaches 50%. It is important to note that although patients with Waldenström macroglobulinemia are at increased risk of infection with the same organisms as asplenic patients, they do not suffer from the other complications of true asplenia, such as overwhelming postsplenectomy infection. Armed with the understanding of this immunologic mechanism, the orthopaedic surgeon will know which patients may be susceptible to infection with encapsulated organisms and may refer such patients to an infectious disease specialist who may optimally manage their immunizations. It is important to remember that although skeletal manifestations of Waldenström macroglobulinemia include conversion to higher-grade lymphomas, these patients have an increased risk for infection with encapsulated organisms, and it must be considered in the differential diagnosis. In this instance, our patient was susceptible not only because the chemotherapy caused a global immunosuppression but also because she had low levels of normal immunoglobulins and an impaired ability to develop immunity despite her up-to-date immunizations. For this reason, we recommend a thorough history and physical examination and appropriate laboratory testing including leukocyte count, C-reactive protein level, and erythrocyte sedimentation rate (which may be elevated at baseline because of the high levels of paraprotein seen in patients with Waldenström macroglobulinemia); appropriate radiographic evaluation; and biopsy of affected areas for both pathology and complete Gram stain and culture regardless of the level of suspicion for infection. This allows the clinician to accurately differentiate and target therapy for these two distinct clinical entities that may present similarly as localized musculoskeletal pain.
Reference: Fabricant PD, Unnanuntana A, Hartman BJ, Lane JM. Multifocal osteomyelitis with Streptococcus pneumoniae in a patient with Waldenström macroglobulinemia. A case report. J Bone Joint Surg Am. 2011;93:e46(1-5).
Non-Hodgkin lymphoma
Hodgkin lymphoma
Bacterial osteomyelitis
Fungal infection

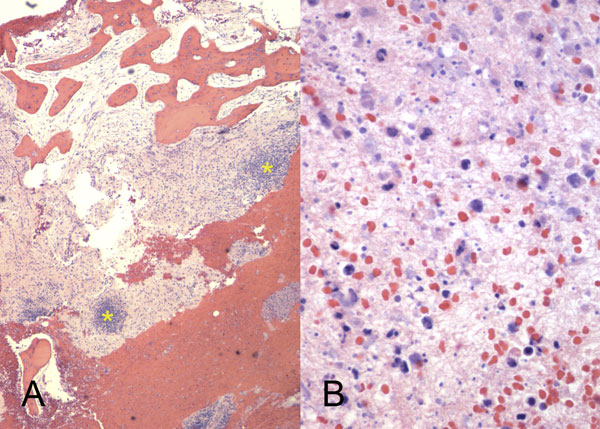
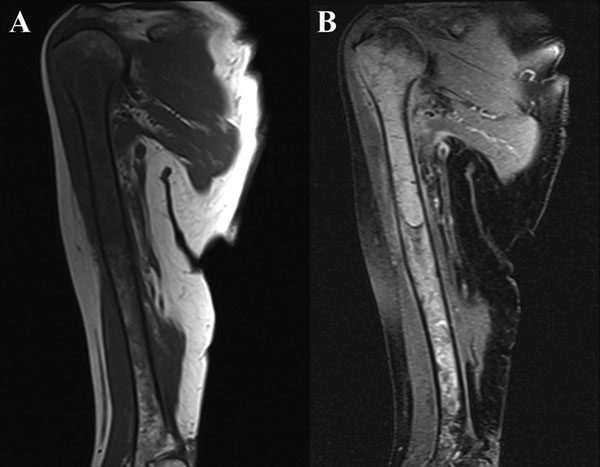
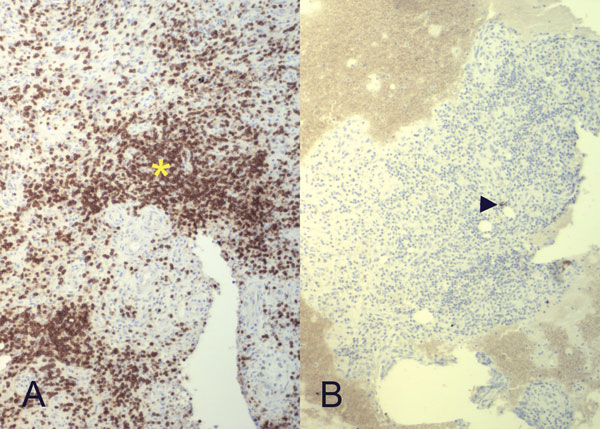
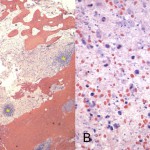 Fig. 2
Fig. 2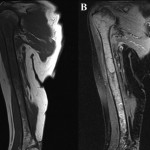 Fig. 1
Fig. 1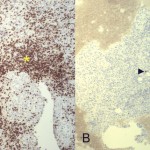 Fig. 3
Fig. 3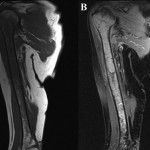 1
1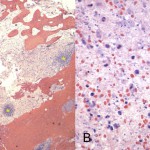 2
2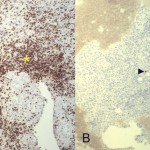 3
3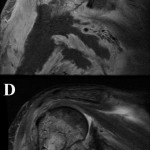 4
4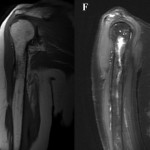 5
5