A Sixty-one-Year-Old Man with History of a Cervical Spine Lesion
April 4, 2012
Twenty years earlier, when he was forty-one years old, this man presented for evaluation of six months of neck pain and two weeks of leg pain. He was otherwise healthy. He denied having had numbness, weakness, paresthesias, or bowel or bladder incontinence. No skin lesions or soft-tissue masses were noted. There was no limitation of neck motion and no visible local mass or tenderness. The findings of the neurologic examination were unremarkable. Imaging studies obtained at presentation included bone scintigraphy demonstrating isolated uptake at the C2 vertebra as well as radiographs, computed tomographic scanning (CT), and magnetic resonance imaging (MRI) demonstrating an expansile, destructive lesion involving both the anterior and the posterior elements of the vertebral body with slight narrowing of the spinal canal and no involvement of surrounding structures (Fig. 1). A halo was placed preoperatively. Intraoperatively, the posterior elements of C2 were distorted and soft and were removed. Frozen section histological evaluation did not show evidence of malignancy. A posterior spinal fusion was performed from C1 to C3 with use of two corticocancellous grafts from the posterior iliac crest, which were stabilized with use of a sublaminar wire at C1 and two spinous process wires at C3. The final histological specimen is shown in Figure 2. Osseous fusion was evident at fourteen weeks. The postoperative clinical course was unremarkable. At the present time twenty years after his cervical fusion, the patient was pain-free without any neurologic deficit or disability. He noted only a mild loss of rotational movement of the cervical spine. Routine follow-up radiographs demonstrated an expansile fusion mass extending to involve C3 (Fig. 3). Subsequent MRI similarly demonstrated extension of the lesion to C2 through the fusion mass into the posterior elements of C3 (Fig. 4). An MR angiogram showed no compression of the vertebral arteries. Since he had few symptoms, he was scheduled for further serial follow-up evaluation and imaging studies.
Reference: Meredith DS, Healey JH. Twenty-year follow-up of monostotic fibrous dysplasia of the second cervical vertebra. A case report and review of the literature. J Bone Joint Surg Am. 2011;93:e74(1-7).
In our literature review, fifty-four cases of monostotic fibrous dysplasia involving the spine were identified. The cases were evenly distributed between males and females and occurred most commonly in the third to fifth decade of life (age range of eleven to sixty-eight years). Patients most commonly presented with axial neck or back pain or with an incidental finding, but more severe presentations, including pathologic fracture of C3 and progressive lower-extremity paresis, have been reported. Of forty-two cases in which a specific location was identified, fifteen (36%) were in the cervical spine, ten (24%) were in the thoracic spine, thirteen (31%) were in the lumbar spine, and four (10%) were in the sacrum. As a result of the rarity of this disorder, the large majority of studies included the results of a single case with a limited duration of follow-up. Consequently, the natural history and ideal treatment of this disease are poorly understood. A unique element of the present case is the demonstrated progression of monostotic fibrous dysplasia into a previously uninvolved vertebra via a remodeled bone autograft. There are several possible explanations for this phenomenon, each of which may provide new insight into the pathophysiology of monostotic fibrous dysplasia. The etiology of fibrous dysplasia has been linked to a sporadic activating mutation of Gs alpha on chromosome 20q13.2-13.3 that occurs after fertilization in somatic cells and results in blocked maturation of primitive bone. This creates a tissue mosaic. Indeed, there is a cellular mosaic of mutated and nonmutated osteoblasts and mesenchymal stem cells in the lesional tissue. Consequently, the clinical presentation of fibrous dysplasia depends on the stage in embryogenesis at which the mutation occurs. Many unaffected areas of cortical bone and circulating blood cells in patients with the polyostotic fibrous dysplasia of McCune-Albright syndrome share the GNAS mutation. In the setting of monostotic fibrous dysplasia, the mutated cells are thought to be limited to the affected bone. Although additional areas may be affected genetically, they fail to establish clinical disease because of the competitive growth disadvantage compared with the nonmutated cells and increased rates of apoptosis of the mutated cells. This same phenomenon is responsible for dampening the activity in monostotic fibrous dysplasia lesions with age and for reports that the histologic characteristics may even normalize. Reactivation and growth of the mutated monostotic fibrous dysplasia cells from C2 must have occurred in our patient, even though he was a middle-aged man. This is a new insight into the behavioral potential of monostotic fibrous dysplasia cells. Remodeling of the bone graft likely provided a conduit for the mutated cells and a mechanism for extension of the disease. Remodeling of cancellous bone graft begins with invasion of the graft marrow space by host fibrovascular reparative tissue, and this is followed by deposition of new bone-encasing graft trabeculae. Remodeling of a cortical graft begins with bridging callus formation from the host periosteum. However, in monostotic fibrous dysplasia the host cell has a genetic block to maturation, and the graft is converted into more fibrous dysplasia. In this case, the graft appears to have served as a bridge for fibrous dysplasia cells to reach a previously unaffected bone, and the remodeling process created an environment that preferentially favored the mutated cells over the normal stromal cells. Another possibility is that C3 or the autologous bone graft could have already been involved by fibrous dysplasia, and represent unrecognized polyostotic fibrous dysplasia. However, this is very unlikely. Among a National Institutes of Health cohort of patients with fibrous dysplasia followed with sequential bone scans, none developed new disease after the age of twenty-three. Our patient’s initial bone scan showed isolated disease of C2. The treatment of spinal fibrous dysplasia is controversial. Several reports describe aggressive excision of monostotic fibrous dysplasia involving the spine in the absence of compression of the neural elements. This approach may be due to concern about potential regrowth of fibrous dysplasia and the risk of malignant transformation of residual disease. Invasion of graft sites by residual fibrous dysplasia has been demonstrated in the appendicular skeleton at rates as high as thirteen (50%) of twenty-six cases. However, good outcomes have been reported in all cases of monostotic fibrous dysplasia of the spine in the literature regardless of the therapeutic intervention used, even with demonstrated invasion of the graft by fibrous dysplasia. Of forty cases for which the outcome of treatment was reported, eleven (28%) were managed with biopsy and observation, ten (25%) were managed with curettage and grafting or fusion, and nineteen (48%) were managed with complete excision with or without fusion on the basis of the resulting degree of spinal instability. All patients had a favorable outcome, and no factors favored a particular approach to treatment. Despite upregulated growth of the neoplastic cells, our patient did not develop cancer. The general risk of malignant transformation of spinal fibrous dysplasia is unknown. We found one case report of monostotic fibrous dysplasia of the spine changing into osteogenic sarcoma, but this one case is insufficient to estimate the rate of malignant transformation. Malignant transformation of fibrous dysplasia is rare in the appendicular skeleton, with a reported incidence of 0.4 to 4%. Our patient will continue to undergo cancer surveillance consistent with our recommendation to perform repeat imaging studies of patients with monostotic fibrous dysplasia every five years because of the risk of malignant transformation. In summary, monostotic fibrous dysplasia of the spine is a rare disorder. Reported presentations and treatments vary, and outcomes have been uniformly good. Thus, it seems prudent to avoid overly aggressive treatment. This case demonstrates the ability of monostotic fibrous dysplasia to spread to a previously uninvolved vertebra through the remodeling of bone graft used for spinal fusion.
Reference: Meredith DS, Healey JH. Twenty-year follow-up of monostotic fibrous dysplasia of the second cervical vertebra. A case report and review of the literature. J Bone Joint Surg Am. 2011;93:e74(1-7).
Paget disease
Fibrous dysplasia
Benign fibrous histiocytoma
Osteoblastoma


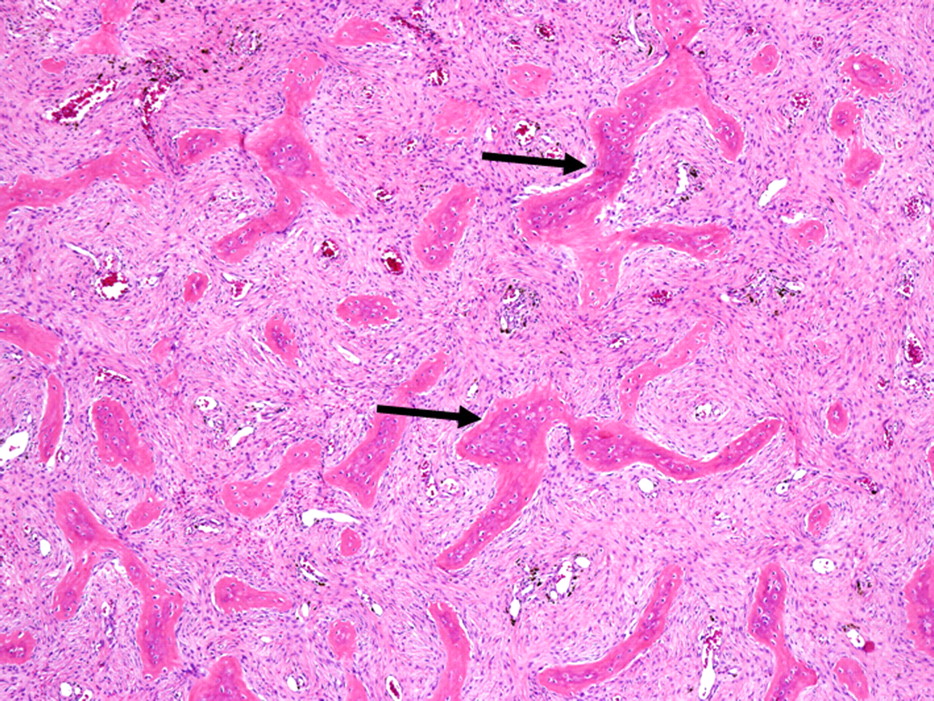
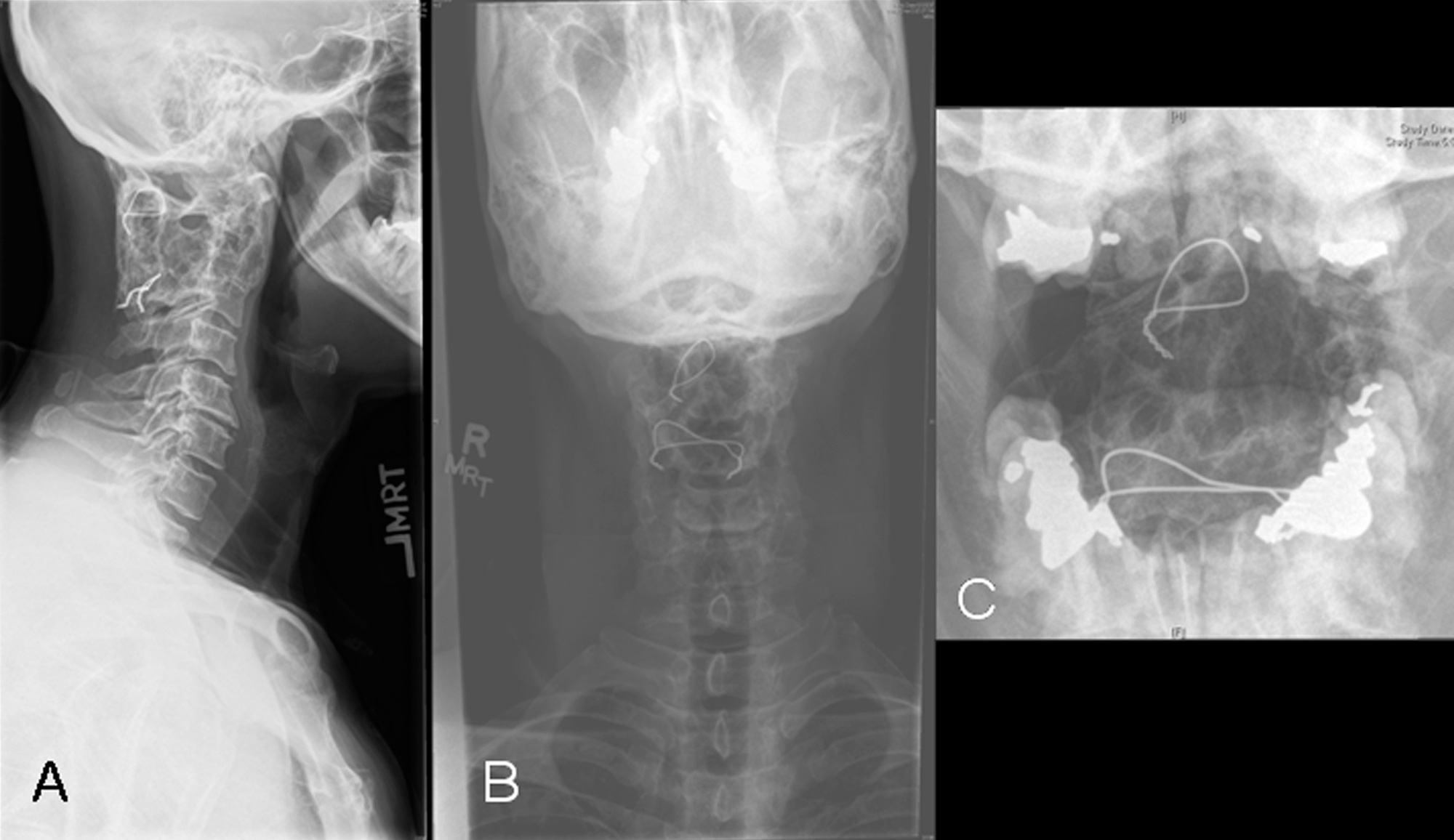
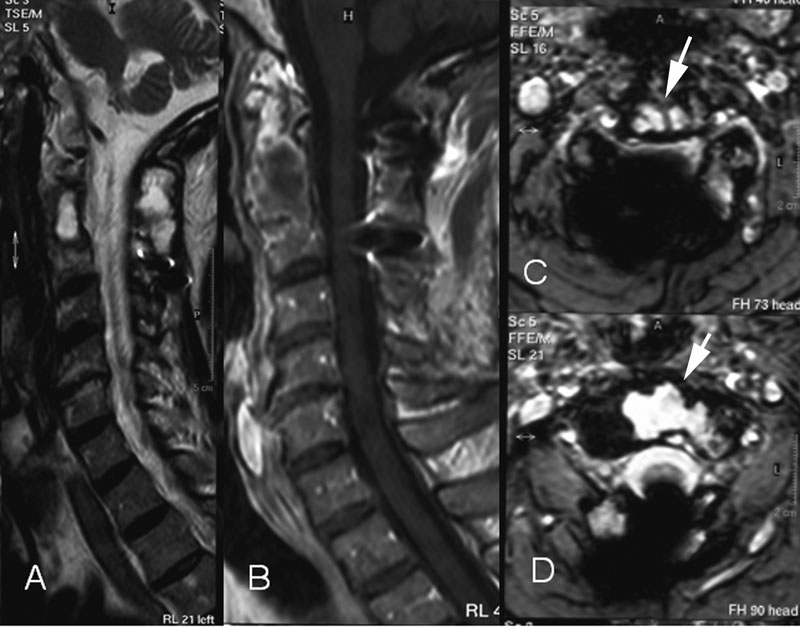
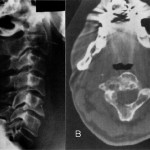 Fig. 1
Fig. 1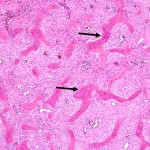 Fig. 2
Fig. 2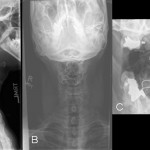 Fig. 3
Fig. 3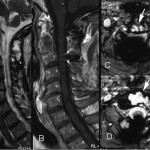 Fig. 4
Fig. 4