A Sixty-three-Year-Old Man with Pain and Swelling of the Left Shoulder
November 5, 2014
A sixty-three-year-old man with rheumatoid arthritis presented with a six-month history of pain and swelling of the left shoulder. Medications included methotrexate (12.5 mg weekly), leflunomide (30 mg daily), and prednisone (5 mg daily). He had no history of tumor necrosis factor antagonist therapy. A review of systems was noteworthy for fatigue, joint pain, stiffness, and swelling; he denied chest pain, cough, shortness of breath, fevers, chills, or night sweats. Physical examination revealed a temperature of 36.6°C and a nontender mass over the anterior aspect of the left shoulder; there was no overlying warmth or erythema. The peripheral blood leukocyte count was normal at 8800 cells/mm with a polymorphonuclear (PMN) predominance (83%); the remainder of the complete blood-cell count and comprehensive metabolic panel was within normal limits. Erythrocyte sedimentation rate (ESR) was elevated at 65 mm/h, and the C-reactive protein (CRP) level was elevated at 36.5 mg/L. Magnetic resonance imaging (MRI) of the left shoulder following intravenous gadolinium contrast administration revealed an enhancing mass involving the rotator cuff musculature, soft tissue, and adipose tissue. The mass was predominantly extra-articular, but intra-articular enhancement was also noted. The long head of the biceps tendon was completely torn, and there was circumferential labral tearing and degeneration (Figs. 1-A through 1-D). Positron emission tomography revealed markedly increased activity matching the areas of the abnormal increased T2-weighted signal seen on MRI. These findings were consistent with either an inflammatory or a neoplastic process; however, a neoplastic process was favored given that the abnormalities were unchanged from the MRI that had been performed two weeks prior. Chest radiography revealed multiple calcified granulomas within the left lung as well as calcified mediastinal lymphadenopathy on the left side.
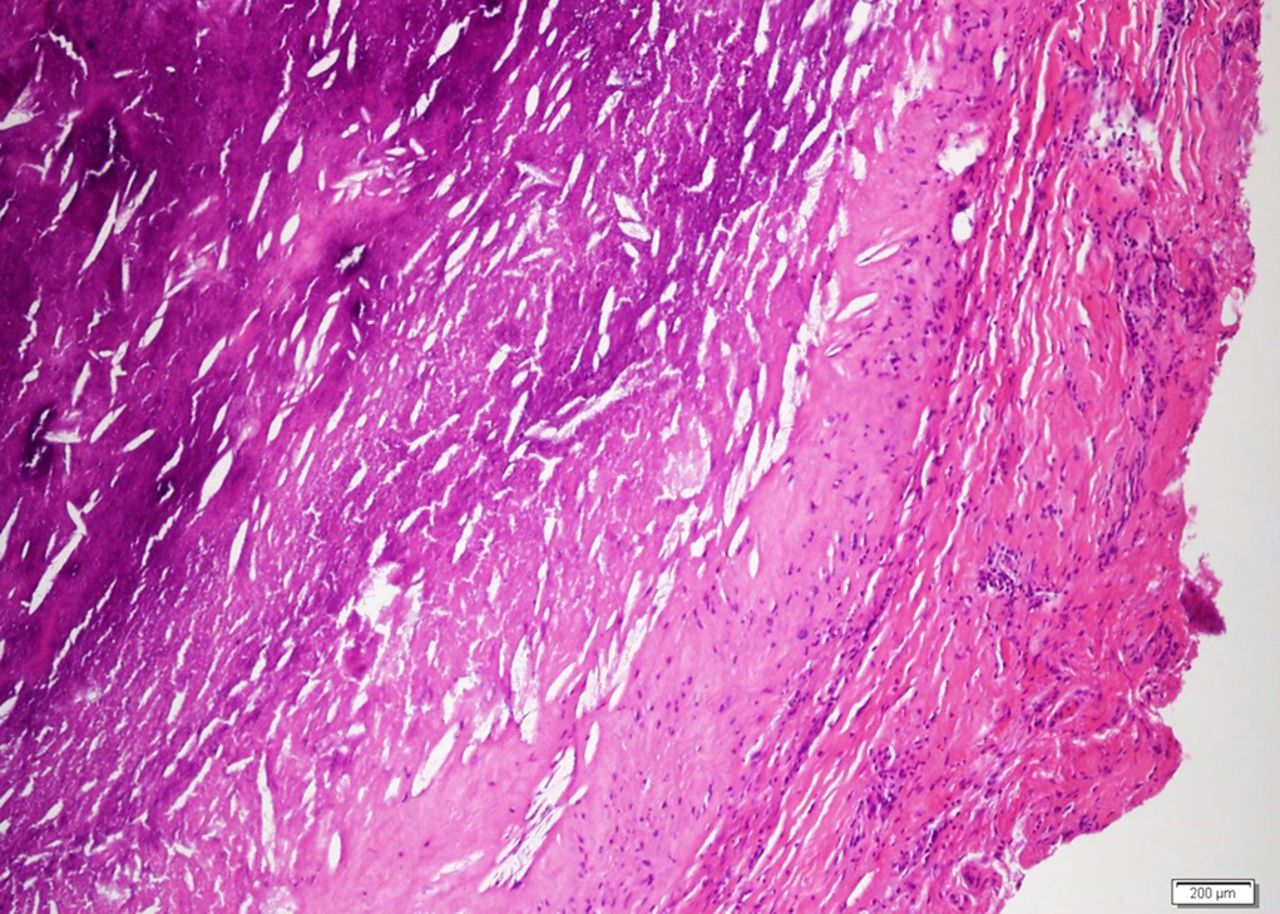
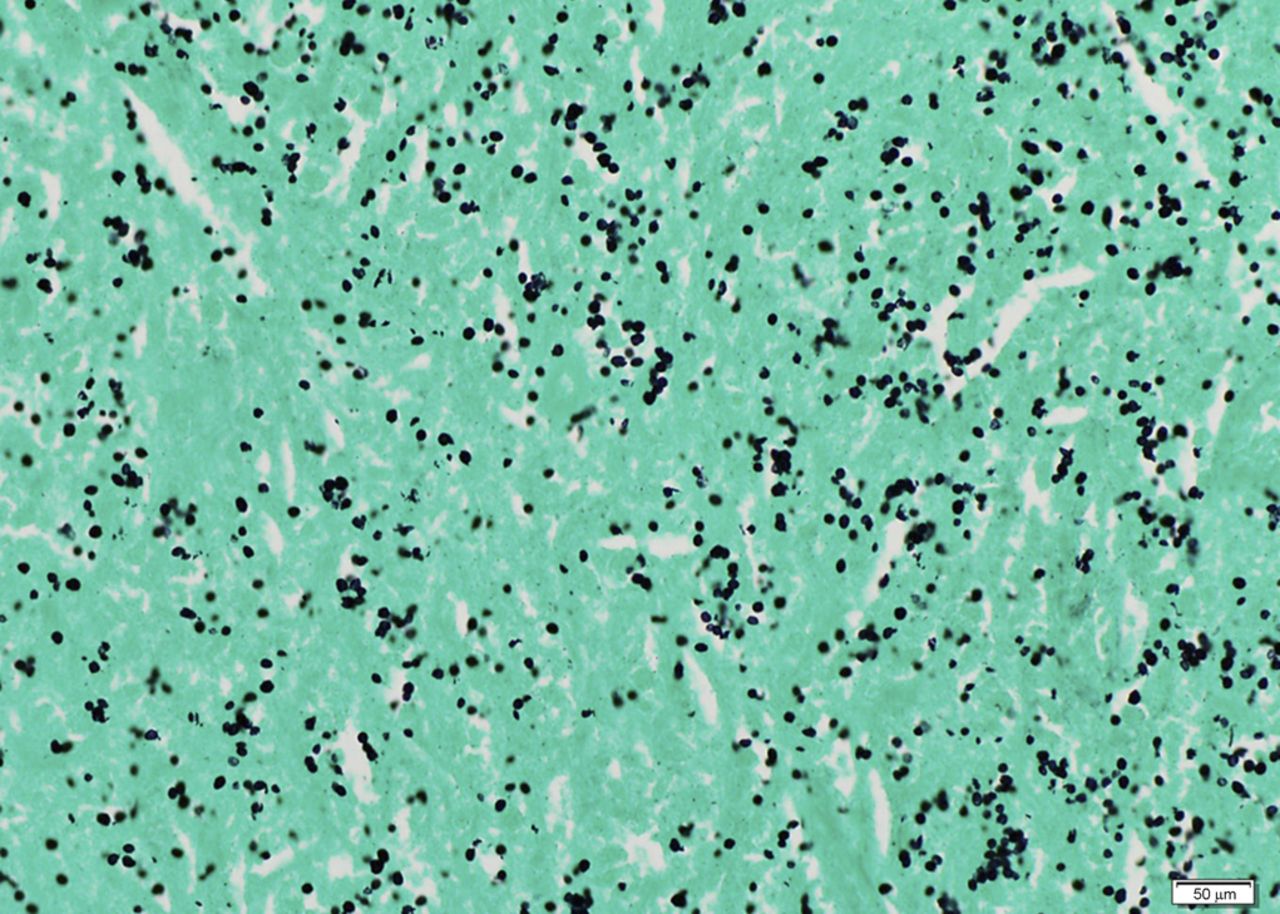
The patient was taken to the operating room for excision of the mass. On visual inspection, the mass was solid, not cystic, and yellow-white in color; an incisional biopsy was performed. The intraoperative frozen section was consistent with a rheumatoid nodule or an atypical infection rather than a neoplasm. A subtotal synovectomy was performed because a total synovectomy is difficult to execute. A portion of the specimen was sent for permanent section, and another portion was preserved for future studies. Intraoperative findings included joint communication and extension of the mass through the ruptured long head of the biceps tendon. Pathologic examination of the permanent section revealed necrotizing granulomatous inflammation (Fig. 2); therefore, additional microbiologic staining and cultures were performed. Bacterial Gram staining revealed 1+ PMN cells, but no organisms. Gomori methenamine silver (GMS) staining revealed numerous yeast forms that are morphologically consistent with Histoplasma species (Fig. 3). Acid-fast bacilli (AFB) stains were negative. Bacterial and AFB cultures were negative. Fungal culture revealed light growth of H. capsulatum. A urine histoplasma antigen was not detectable by enzyme immunoassay (MiraVista Diagnostics, Indianapolis, Indiana). The patient was started on itraconazole solution (200 mg twice daily with cola). The shoulder pain and swelling worsened, and subsequent itraconazole and hydroxyitraconazole levels were undetectable (MiraVista Diagnostics) despite an increase in dosage. Therefore, the itraconazole was discontinued and the patient was started on voriconazole (400 mg twice daily). At this dose, voriconazole levels were maintained at greater than 2 µg/mL (MiraVista Diagnostics). After six months of therapeutic drug levels, the left shoulder symptoms had resolved, and the voriconazole was discontinued because of its side effects. Almost four years later, the left shoulder mass recurred. At that time, the methotrexate and leflunomide had been discontinued, and the prednisone dose had been increased to 5 mg twice daily. The peripheral blood leukocyte count was normal at 9600 cells/mm without PMN predominance (58%), the ESR was lower but still elevated at 58 mm/h, and the CRP level was normal at 1.9 mg/L. Pathologic examination of a fine-needle aspirate revealed macrophages and fungal elements that were morphologically consistent with Histoplasma species. The patient was taken back to the operating room for an arthrotomy and a synovectomy. Pathologic tissue examination again revealed necrotizing granulomatous inflammation and numerous yeast forms that were morphologically consistent with Histoplasma species. Fungal culture revealed light growth of H. capsulatum. Given the patient’s previous side effects on voriconazole, he was started on posaconazole (400 mg twice daily with fatty food). Serum posaconazole levels remained higher than 1 µg/mL (National Medical Services, Willow Grove, Pennsylvania). The patient completed approximately twelve months of therapy without complications and with improvement in symptoms.
Proceed to Discussion >>Reference: Pettit AC, Raynor, MB, Schwartz HS, Wright PW. Histoplasmosis masquerading as a rheumatoid nodule in an immunocompromised host. A case report. JBJS Case Connect, 2014 Sep 10;4(3):e75.
Histoplasmosis is endemic in the Ohio and Mississippi River Valleys of the United States, as well as in parts of South America, Africa, and Asia. Exposure occurs through inhalation of microconidia from an endemic environment. As with this case, some patients will not experience any respiratory illness following exposure, while others may experience severe pneumonitis and respiratory failure. Pulmonary histoplasmosis may lead to findings such as calcified pulmonary granulomas and calcified mediastinal lymphadenopathy, which were identified in our patient by chest radiography. During the acute phase of infection, hematogenous dissemination will occur in most patients. However, in immunocompetent persons, progressive disseminated disease is rare because of the development of cell-mediated immunity. Progressive disseminated disease is defined as longer than three weeks of clinical illness in the setting of physical examination or radiographic findings and/or laboratory evidence of extrapulmonary disease. Laboratory evidence includes the identification of granulomas with yeast forms that are morphologically consistent with H. capsulatum or growth of H. capsulatum in culture from extrapulmonary tissues with or without antigenuria and/or antigenemia. Up to 30% to 50% of patients with disseminated disease may not have concurrent lung involvement. Arthritis or arthralgia during acute pulmonary histoplasmosis may occur as a systemic inflammatory response, but does not represent active disseminated infection in the joints. Bone or joint infection is a rare extrapulmonary manifestation of active progressive disseminated histoplasmosis. A review published in 2001 revealed only eight culture-confirmed cases of musculoskeletal histoplasmosis. Histoplasmosis of the joints occurs most commonly in the knee and in the joints of the hand. Recommended stains for the identification of fungal elements are the GMS and periodic acid-Schiff stains. Histoplasma yeast is characteristically 3 to 5 µm in diameter and ovoid in shape with narrow-based budding. However, the morphology is not specific, and misidentification may occur in the absence of an experienced pathologist. Among patients with disseminated disease, positive histopathology and cytolopathology occur in 18% to 62% of cases. The rate of culture positivity of extrapulmonary tissue in the setting of disseminated histoplasmosis is 74% to 82%. Urine histoplasma antigen can be detected in over 90% of patients with disseminated disease. Antigen levels have been shown to decrease during therapy and to increase in the majority of patients who relapse. Therefore, it is recommended that antigen levels be measured prior to treatment initiation, during therapy, and at the time when treatment failure or relapse is suspected. The urine antigen may be undetectable in up to 25% of patients with disseminated histoplasmosis who do not have acquired immune deficiency syndrome. Among these patients, the peripheral blood leukocyte count with the proportion of PMN cells, the ESR, or the CRP level may be useful adjunctive laboratory tests to follow during therapy. Oral itraconazole is the preferred agent for treatment of mild-to-moderate disseminated disease for at least twelve months’ duration. The response rate with itraconazole therapy for mild-to-moderate disseminated histoplasmosis is 80% to 100%. Difficulty in treatment usually arises from drug intolerance, inability to achieve adequate drug levels, or concomitant serious drug-drug interactions. Gastrointestinal intolerance occurs in upward of 10% of patients. The inability to achieve adequate drug levels varies depending on the drug formulation, the presence of food in the stomach, the acidity of the stomach, and concomitant medications. It is recommended that serum drug levels be obtained at steady state (after two weeks of therapy). The sum of itraconazole and its bioactive metabolite, hydroxyitraconazole, should be greater than 1.0 µg/mL. All available azoles are alternatives to itraconazole. Fluconazole may be less effective than itraconazole, and ketoconazole is rarely used in the U.S. because of its toxicity. Voriconazole and posaconazole have in vitro activity against H. capsulatum, and both have been used with success. Relapse of histoplasmosis occurs in 10% to 15% of cases, but is rare in the setting of adequate therapy and the development of cell-mediated immunity. Lifelong suppressive therapy with itraconazole may be considered in immunocompromised patients if the immunosuppression cannot be reversed or in those who relapse despite appropriate therapy. In our patient, disease recurred at the same unusual location, which was likely due to relapse in the setting of inadequate surgical debridement and/or antifungal therapy. This case demonstrates an unusual presentation of progressive disseminated histoplasmosis infection leading to a periarticular shoulder mass. Histoplasmosis should be considered in the differential diagnosis of a soft-tissue mass that is identified in an immunocompromised patient from an endemic area. Tissue histopathology suggestive of granulomatous inflammation should lead to microbiologic examination, including fungal stains and cultures, to increase the diagnostic yield.
Reference: Pettit AC, Raynor, MB, Schwartz HS, Wright PW. Histoplasmosis masquerading as a rheumatoid nodule in an immunocompromised host. A case report. JBJS Case Connect, 2014 Sep 10;4(3):e75.
Histoplasmosis
Tubercular arthritis of the shoulder
Liposarcoma
Cuff tear arthropathy
Rheumatoid nodule


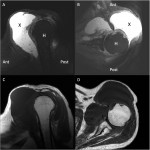 Fig. 1
Fig. 1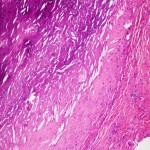 Fig. 2
Fig. 2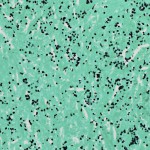 Fig. 3
Fig. 3